Abstract
This study aimed to develop and validate a nomogram model that includes clinical and laboratory indicators to predict the risk of metabolic-associated fatty liver disease (MAFLD) in young Chinese individuals. This study retrospectively analyzed a cohort of young population who underwent health examination from November 2018 to December 2021 at The Affiliated Hospital of Southwest Medical University in Luzhou City, Sichuan Province, China. We extracted the clinical and laboratory data of 43,040 subjects and randomized participants into the training and validation groups (7:3). Univariate logistic regression analysis, the least absolute shrinkage and selection operator regression, and multivariate logistic regression models identified significant variables independently associated with MAFLD. The predictive accuracy of the model was analyzed in the training and validation sets using area under the receiver operating characteristic (AUROC), calibration curves, and decision curve analysis. In this study, we identified nine predictors from 31 variables, including age, gender, body mass index, waist-to-hip ratio, alanine aminotransferase, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, uric acid, and smoking. The AUROC for the subjects in the training and validation groups was 0.874 and 0.875, respectively. The calibration curves show excellent accuracy of the nomogram. This nomogram which was based on demographic characteristics, lifestyle habits, anthropometrics, and laboratory data can visually and individually predict the risk of developing MAFLD. This nomogram is a quick and effective screening tool for assessing the risk of MAFLD in younger populations and identifying individuals at high risk of MAFLD, thereby contributing to the improvement of MAFLD management.
Similar content being viewed by others
Introduction
MAFLD refers to a fatty liver disease resulting from systemic metabolic dysregulation, and is renamed from non-alcoholic fatty liver disease (NAFLD). According to the related statistics, the incidence and prevalence of MAFLD are rapidly increasing globally, which grows currently by about 25% worldwide1.MAFLD is increasingly becoming a disastrous threat to human health.
Due to improved nutrition and a significant shift in dietary habits among young people, the number of cases of obesity in this age group continues to rise2. Meanwhile, the onset of MAFLD is insidious and often asymptomatic in its early stages. Without timely diagnosis and treatment, MAFLD can lead to chronic hepatitis, cirrhosis, and hepatocellular carcinoma. It can also contribute to the progression of other metabolism-related disorders such as diabetes mellitus, hyperlipidemia, and hyperuricemia3,4. Neglecting the health status of the younger population may also lead to the continued progression of MAFLD and result in a significant health burden. Therefore, early detection and management are essential to prevent the progression of MAFLD. By far, among all the available diagnostic tools, liver biopsy is regarded as the gold standard for the diagnosis of MAFLD. Nevertheless, due to its invasive nature, liver biopsy is rarely recommended for routine screening. Other imaging techniques, such as magnetic resonance spectroscopy (MRS) and computed tomography (CT) are neither cost-friendly for mass screening nor user-friendly (difficult to generalize). Ultrasound seems to be widely used as the diagnostic modality for identifying steatosis for its cost and convenience. Yet its accuracy heavily relied on the operator’s personal experience, let alone its sensitivity for detection of < 20% steatosis, all these factors have constrained MAFLD screening in developing or impoverished areas5. Therefore, it is profound to develop a simple, non-invasive, and practical prediction model for rapid and effective screening of MAFLD. Several simple screening tools for MAFLD have emerged based on demographics, laboratory factors, and anthropometrics6,7. However, these models may only focus on the overweight population8 or concentrate solely on the medical population in the United States, thus applicability to Chinese is yet determined9. Consequently, no suitable MAFLD screening tool has been developed for the young Chinese population.
Nomogram is widely used. It is a graphical prediction tool that visually quantifies an event's risk based on the variables’ specificity10. We aimed to develop and validate a nomogram based on routine indicators associated with MAFLD during health examinations, in order to screen for MAFLD in young Chinese populations.
Materials and methods
Participants
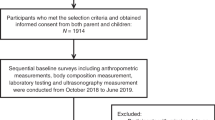
This study retrospectively included a population of young adults (18–44 years old) who underwent health examination at The Affiliated Hospital of Southwest Medical University in Sichuan Province, China, from November 2018 to December 2021, including a total of 43,461 subjects with complete ultrasound data examinations. We obtained Participant demographics, anthropometry, laboratory tests, and personal history data from electronic medical examination systems. The research flow chart is shown in Fig. 1. All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by Ethics Committee of the Affiliated Hospital of Southwest Medical University. Informed consent was waived by the Ethics Committee of the Affiliated Hospital of Southwest Medical University because of the retrospective nature of our study.
Data collection
The collected variables included factors that previous studies have demonstrated to be associated with MAFLD, including demographics (age, sex), anthropometrics (body mass index [BMI], systolic blood pressure [SBP], diastolic blood pressure [DBP], waist circumference [WC], waist-to-hip ratio [WHR]), laboratory tests (neutrophil [NEU], lymphocyte [LYM], white blood cell [WBC], red blood cell [RBC], platelets [PLT], hemoglobin [HGB], alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase [ALP], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], uric acid [UA], total cholesterol [TC], triglyceride [TG], total protein [TP], globulin [GLO], albumin [ALB], total bilirubin [TBIL], direct bilirubin [DBIL], indirect bilirubin [IBIL], creatinine [Crea], glomerular filtration rate [GFR], fasting blood glucose [FPG]), living habits (Smoking).
Our study used the simplified Modification of Diet in Renal Disease (MDRD) formula that was improved by the Chinese to calculate glomerular filtration rate (GFR): GFR (ml/[min × 1.73 m2]) = 186 × (Cr [mg/dl])−1.154 × (age)−0.203 × (0.742 if female)11.
A total of 31 variables were collected.
Definition and assessment
We classified BMI into four groups according to the World Health Organisation’s BMI standards for Asians: underweight: < 18.50 kg/m2,normal: 18.50–22.99 kg/m2,overweight: 23.00–24.99 kg/m2, obese: ≥ 25.00 kg/m212.
After resting for 5 min before the blood pressure measurement, the participant took the right arm blood pressure, including systolic and diastolic blood pressure, in a sitting position. Abnormal systolic blood pressure was defined as systolic blood pressure greater than or equal to 130 mmHg or a previous diagnosis of hypertension, and abnormal diastolic blood pressure was defined as diastolic blood pressure greater than or equal to 85 mmHg or a previous diagnosis of hypertension. We defined WC abnormalities as WC ≥ 90 cm in men and WC ≥ 80 cm in women3. Abnormal WHR was defined as ≥ 0.90 in males and ≥ 0.85 in females13. Dyslipidemia was defined as follows: TC ≥ 5.2 mmol/L; LDL-C ≥ 3.4 mmol/L; HDL-C < 1 mmol/L in men and < 1.3 mmol/L in women; TG ≥ 1.7 mmol/L6. Hyperuricaemia was defined as UA > 420 μmol/L14. Our study defined impaired fasting glucose as FPG ≥ 5.6 mmol/L3.
MAFLD
The diagnosis of hepatic steatosis in this study was based on the results of liver ultrasonography. This institution is a tertiary medical center, trained sonographers performed liver ultrasonography, and the ultrasound diagnosis of hepatic steatosis was based on the presence of hepatic and renal echocontrast, liver parenchymal brightness, deep attenuation, and vascular blurring15. The diagnosis of MAFLD is based on ultrasound-confirmed hepatic steatosis and one of the following three criteria: overweight or obesity, presence of type 2 diabetes, or metabolic dysfunction. Metabolic dysfunction requires at least two conditions to be met: (1) male WC ≥ 90 cm, female WC ≥ 80 cm; (2) blood pressure ≥ 130/85 mmHg or specific drug treatment; (3) hypertriglyceridemia (TG ≥ 1.70 mmol/L or on lipid-lowering therapy); (4) HDL-C < 1.0 mmol/L in men, < 1.3 mmol/L in women; (5) prediabetes3.
Statistical analysis
For model development, all participants were randomly divided into two groups in a 7:3 ratio for training and validation using the “caret” package. We used the training dataset to develop the model and the validation dataset for internal validation. Then, we assessed the comparability between the two datasets. We selected the t-test or Wilcox rank-sum test for continuous variables based on homogeneity of variances and normality. We selected the chi-squared test or Fisher’s exact test for categorical data (dichotomous and multichotomous) depending on the data condition. Univariate logistic regression analysis was used to identify potential predictors of MAFLD. To ensure that the univariate logistic regression model was not overfitting, LASSO was performed to eliminate factors with high correlation. In subsequent analyses, independent predictors were further identified using multivariate logistic regression. This study then developed a nomogram to predict MAFLD risk using the “rms” package. ROC was used to assess discriminant performance, with an AUROC greater than 0.70 reflecting the high performance of the nomogram. A calibration curve is used to measure the agreement between actual outcomes and predicted probabilities. The clinical utility of the nomogram was assessed by DCA.
Statistical analyses were performed using R software version 4.3.1 and SPSS version 24.0. A two-sided P value < 0.05 was considered statistically significant.
Ethical approval
The study was reviewed by the Ethics Committee of the Affiliated Hospital of Southwest Medical University.
Results
Missing values for variables
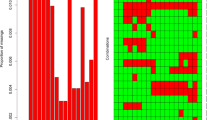
A missing value test was shown on the collected data, and 1831 missing values were detected. Figure 2 shows missing data for 31 variables. Bar graphs show missing proportions for all variables below 0.6%.
Study cohorts
Participants were randomly divided into training groups (n = 30,128) and validation groups (n = 12,912) in a ratio of 7:3. There were no statistically significant differences in clinical information or laboratory results between the two cohorts (P > 0.05). The characteristics of the two datasets are shown in Supplementary Table 1.
Selection of major predictors for MAFLD
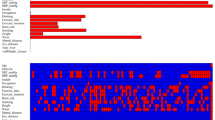
Table 1 shows the results of univariate logistic regression analysis. Univariate logistic regression analysis identified 17 candidate characteristics, including age, gender, BMI, SBP, DBP, WC, WHR, ALT, AST, LDL, HDL, UA, TC, TG, Crea, FPG, smoking. To ensure that the univariate logistic regression model does not overfit, LASSO was performed to eliminate highly correlated factors, resulting in 10 variables with non-zero coefficients, including age, gender, BMI, WHR, ALT, LDL, HDL, UA, TG, smoking (Fig. 3). Next, we use multivariate logistic regression analysis to identify independent factors closely related to MAFLD. The results showed nine variables could be used as independent predictors of MAFLD (Table 2), including age, gender, BMI, WHR, ALT, LDL, HDL, UA, and smoking.
Nomogram construction
A nomogram was established on the basis of the results of multivariate logistic regression analysis to predict the risk of MAFLD with age, gender, BMI, WHR, ALT, LDL, HDL, UA, and smoking (Fig. 4). A score for that variable is obtained based on the scale corresponding to the top of each predictor variable. The total score is the sum of the individual scores. When the total score corresponds vertically down the scale to the likelihood of diagnosis, the risk of each individual can be calculated.
Verification and calibration of nomogram
ROC curves were used to assess the discriminative power of the nomogram models. The AUROC for the training and validation groups are 0.874 and 0.875, respectively (Supplementary Fig. 1).
In calibration curves, the dashed lines represent the ideal model and the solid lines represent the predictive performance of the nomogram. The closer the distance between the two lines, the better the performance of the nomogram. The overall calibration ability was good in our model (supplementary Fig. 2).
The DCA of the nomogram is shown in Supplementary Fig. 3. Decision curves showed that the model provided a more significant net gain in predicting the risk of developing MAFLD than the “all” or “none” strategy when the individual’s threshold probability was in the range of approximately 0.03–0.83.
Discussion
Health examination has increasingly become a mean of public health management with the enhancement of health awareness. Due to the insidious onset of MAFLD, there is a lack of specific clinical symptoms and signs16. Therefore, MAFLD is often diagnosed incidentally during a health examination. Awareness and concern about MAFLD are generally low in the younger population. Research has found that weight loss, calorie restriction, and exercise may reverse the process of MAFLD in its early stages17. Accordingly, given the impact of MAFLD on health, early detection and intervention, particularly among young people, may play a key role in reducing the future national health burden.
This study based on the data from health checkups at The Affiliated Hospital of Southwest Medical University, variables were screened by logistic regression and LASSO regression analyses. We finally established a nomogram for predicting the risk of MAFLD in the younger population by using indicators that are easily accessible in various healthcare facilities. Testing of the model revealed our nomogram has good performance in terms of discrimination, calibration, and clinical utility and thus can intuitively predict the risk of MAFLD in the young population.
The model utilises easily accessible variables, such as demographic characteristics (age, sex), lifestyle habits (smoking history), anthropometrics (BMI, WHR), and laboratory data (ALT, LDL, HDL, and UA).
The correlation between demographic characteristics and MAFLD has been reported in previous studies. The risk of MAFLD increases with age. A study by Lazo M et al. showed the incidence of MAFLD is 16.1% in the 30–40 age group, 22.3% in the 41–50 age group, 29.3% in the 51–60 age group, and 27.6% in the 60 + age group18.
Our study also found a higher risk of MAFLD in men compared to women, which is similar to previous studies. Although the mechanism is still unclear, some researchers believe that estrogen deficiency will exacerbate liver inflammation and accelerate the progression of the disease19.
Regarding lifestyle, our study found smoking to be an independent predictor of MAFLD. Epidemiological evidence on the association of smoking with MAFLD has shown conflicting results in previous studies. In a cross-sectional study of 90 patients with MAFLD, no association was found between smoking and MAFLD20. Another cross-sectional study of 2811 participants found a 1% increased risk of MAFLD among those who smoked an additional pack of cigarettes per day21. Potential mechanisms underlying the association between smoking and MAFLD may be related to insulin resistance, hyperinsulinemia, dyslipidemia, hepatic steatosis, inflammation, and elevated catecholamine and glucagon levels22.
There is an international consensus on the relevance of anthropometry and MAFLD. Multiple studies have shown a strong relationship between obesity and MAFLD. BMI and WHR are commonly used to assess overweight or obesity, which has been included in the diagnostic criteria for MAFLD. Their risk has been confirmed in several studies23,24. However, recent years, studies have found that the incidence of MAFLD in the non-obese population is rising. Ye et al. showed that about 40% of patients with MAFLD are non-obese, and nearly one-fifth are lean. Both the non-obese and lean groups had substantial long-term hepatic and non-hepatic comorbidities25. Indications reveal that obesity should not be the only criterion for MAFLD, we should also pay enough attention to those with normal or low BMI and WHR.
In our study, four laboratory indicators were independent predictors of MAFLD, including ALT, LDL, HDL, and UA. ALT is a liver enzyme found primarily in the liver and is commonly used to indicate the presence of liver disease, which is a specific marker of liver inflammation and hepatocellular injury26. However, some researchers believe that a strict ALT threshold is needed to evaluate the occurrence and development of MAFLD, inaccurate ALT threshold may lead to an underestimation of liver injury27. Abnormal LDL and HDL levels reflect abnormalities in lipid metabolism, strongly associated with MAFLD28. Our study presented similar results. Souza et al. have shown that low levels of HDL-C are a risk factor for MAFLD. Low HDL-C levels may be caused by physical inactivity, obesity, and diabetes29. Through the cholesterol reverse transport pathway, HDL-C can promote dietary cholesterol’s efflux to exert anti-inflammatory and antioxidant effects30. Elevated levels of UA are commonly observed in patients with metabolic syndrome. In our study, high UA level is an independent predict factor for the risk of MAFLD, which is similar to previous studies31. However, some researchers argue that while previous studies have demonstrated a correlation between hyperuricemia and MAFLD, establishing a causal relationship is challenging32.
In summary, our study used readily available clinical and laboratory data to construct a nomogram model, which could predict the risk of MAFLD in the young Chinese population. This model suggests that MAFLD can be prevented by reducing fat, losing weight, and smoking cessation, which is significant for disease prevention and health care of young people. In addition, our nomogram model can support clinicians in screening for MAFLD and determining whether patients need further abdominal ultrasonography to confirm the diagnosis of MAFLD. Meanwhile, it can provide self-management for potentially at-risk patients with MAFLD to seek timely medical attention.
Limitations
There are also some limitations of our study. Firstly, this study was a single-center retrospective study, which may introduce bias. Furthermore, previous studies have suggested that potential factors such as exercise, sleep, anxiety-depression scores, and dietary patterns are strongly associated with the development of MAFLD. However, we were unable to obtain this data from the medical examiners and therefore cannot provide recommendations for further lifestyle modifications. Additionally, although our sonographers have received professional training, liver biopsy remains the gold standard for the diagnosis of MAFLD. However, it is impractical to carry out liver biopsy in a large-scale medical examination population. Future studies should aim to increase the use of liver biopsy to improve the accuracy of MAFLD diagnosis.
Conclusions
By analyzing demographic characteristics, living habits, anthropometry, and laboratory data, this study constructed a nomogram for predicting the risk of MAFLD in young people. Healthcare professionals can use this model to analyze the risk of MAFLD in patients and provide individualized prevention and treatment plans based on risk assessment. Early intervention to prevent disease progression may reduce the risk of adverse outcomes.
Data availability
No datasets were generated or analysed during the current study.
References
Huang, D. Q., El-Serag, H. B. & Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 18(4), 223–238 (2021).
Zhang, X. et al. Increasing prevalence of NAFLD/NASH among children, adolescents and young adults from 1990 to 2017: A population-based observational study. BMJ Open 11(5), e042843 (2021).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73(1), 202–209 (2020).
Sarin, S. K. et al. Liver diseases in the Asia-Pacific region: A lancet gastroenterology & hepatology commission. Lancet Gastroenterol. Hepatol. 5(2), 167–228 (2020).
Maurice, J. & Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. (Lond). 18(3), 245–250 (2018).
Zhou, B. et al. Development and validation of a nomogram for predicting metabolic-associated fatty liver disease in the Chinese physical examination population. Lipids Health Dis. 22(1), 85 (2023).
Lee, J.-H. et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42(7), 503–508 (2010).
Song, D. et al. Development and validation of a nomogram for prediction of the risk of MAFLD in an overweight and obese population. J. Clin. Transl. Hepatol. 10(6), 1027–1033 (2022).
Zou, H., Zhao, F., Lv, X., Ma, X. & Xie, Y. Development and validation of a new nomogram to screen for MAFLD. Lipids Health Dis. 21(1), 133 (2022).
Wang, X. et al. From past to future: Bibliometric analysis of global research productivity on nomogram (2000–2021). Front. Public Health. 10, 997713 (2022).
Li, L., Cheng, S. & Xu, G. Identification of risk factors for hypertension in overweight and obese people and analysis of risk factor interactions: An R-based analysis. Front. Cardiovasc. Med. 10, 1180698 (2023).
Khuon, D. et al. BMI as a predictor of high fasting blood glucose among people living with HIV in the Asia-Pacific region. HIV Med. 24(2), 139–152 (2023).
Ren, H., Guo, Y., Wang, D., Kang, X. & Yuan, G. Association of normal-weight central obesity with hypertension: A cross-sectional study from the China health and nutrition survey. BMC Cardiovasc. Disord. 23(1), 120 (2023).
Zhang, M. et al. Prevalence of hyperuricemia among Chinese adults: Findings from two nationally representative cross-sectional surveys in 2015–16 and 2018–19. Front. Immunol. 12, 791983 (2021).
Dietrich, C. F. et al. Conventional ultrasound for diagnosis of hepatic steatosis is better than believed. Z. Gastroenterol. 60(8), 1235–1248 (2022).
Aguilera-Méndez, A. Nonalcoholic hepatic steatosis: A silent disease. Rev. Med. Inst. Mex. Seguro. Soc. 56(6), 544–549 (2019).
Nassir, F. NAFLD: Mechanisms, treatments, and biomarkers. Biomolecules 12(6), 824 (2022).
Lazo, M. et al. Prevalence of nonalcoholic fatty liver disease in the United States: The third national health and nutrition examination survey, 1988–1994. Am. J. Epidemiol. 178(1), 38–45 (2013).
Ballestri, S. et al. NAFLD as a sexual dimorphic disease: Role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv. Ther. 34(6), 1291–1326 (2017).
Yilmaz, Y., Yonal, O., Kurt, R. & Avsar, E. Cigarette smoking is not associated with specific histological features or severity of nonalcoholic fatty liver disease. Hepatology 52(1), 391 (2010).
Koehler, E. M. et al. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J. Hepatol. 57(6), 1305–1311 (2012).
Jung, H.-S. et al. Smoking and the risk of non-alcoholic fatty liver disease: A cohort study. Am. J. Gastroenterol. 114(3), 453–463 (2019).
VanWagner, L. B. et al. Body mass index trajectories in young adulthood predict non-alcoholic fatty liver disease in middle age: The CARDIA cohort study. Liver Int. 38(4), 706–714 (2018).
Abeysekera, K. W. M. et al. Evaluating future risk of NAFLD in adolescents: A prediction and decision curve analysis. BMC Gastroenterol. 22(1), 323 (2022).
Ye, Q. et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 5(8), 739–752 (2020).
Pratt, D. S. & Kaplan, M. M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 342(17), 1266–1271 (2000).
Kolahdoozan, S. et al. Upper normal limits of serum alanine aminotransferase in healthy population: A systematic review. Middle East J. Dig. Dis. 12(3), 194–205 (2020).
Sheka, A. C. et al. Nonalcoholic steatohepatitis: A review. JAMA 323(12), 1175–1183 (2020).
Souza, M. R. D. A., Diniz, M. D. F. F. D. M., Medeiros-Filho, J. E. M. D. & Araújo, M. S. T. D. Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq. Gastroenterol. 49(1), 89–96 (2012).
Crudele, L. et al. Low HDL-cholesterol levels predict hepatocellular carcinoma development in individuals with liver fibrosis. JHEP Rep. 5(1), 100627 (2023).
Wu, S.-J. et al. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: A large population-based study. Medicine (Baltimore) 94(17), e802 (2015).
Oral, A., Sahin, T., Turker, F. & Kocak, E. Relationship between serum uric acid levels and nonalcoholic fatty liver disease in non-obese patients. Medicina (Kaunas) 55(9), 660 (2019).
Acknowledgements
We thank the Health Management Center of the Affiliated Hospital of Southwest Medical University for providing us with the data. We thank the patients for allowing us to share their clinical data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Y. Y. and M. X. are equally contributing first authors. Y. Y. and M. X. designed the study. Y. Z., X. Y. and X. Z. performed the study and analyzed the data. G. X. and X. T. provided scientific insight to complete the study. Y. Y. and M. X. wrote the first draft of the manuscript and prepared the final version of the manuscript. All authors contributed to data interpretation, writing, and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, Y., Xu, M., Zhang, X. et al. Development and validation of a nomogram model for predicting the risk of MAFLD in the young population. Sci Rep 14, 9376 (2024). https://doi.org/10.1038/s41598-024-60100-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-60100-y
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.