Abstract
To investigate the predictive value of baseline platelet count and its short-term dynamic changes in the prognosis of patients with acute heart failure (AHF) in the intensive care unit. Patients diagnosed with AHF in the medical information mart for intensive care III and their clinical data were retrospectively filtered. Patients were divided into survivor and non-survivor groups based on their prognosis during hospitalization, and differences in baseline data between groups were compared. Logistic regression models and restricted cubic spline (RCS) plots were performed to evaluate the relationship between baseline platelet counts and in-hospital mortality. Changes and trends in platelet counts were compared between the survivor and non-survivor groups after adjusting for confounders with the generalized additive mixing model (GAMM). A total of 2930 critical patients with acute heart failure were included, of which 2720 were survivors and 210 were non-survivors. Multiple logistic regression models revealed that baseline platelet count was an independent factor in hospital mortality (OR 0.997, 95% CI 0.994–0.999, P-value = 0.018). The RCS plot demonstrated a U-shaped dose–response relationship between baseline platelet count and in-hospital mortality. GAMM analysis suggested that the platelet counts decreased and then increased in the survivor group and gradually decreased in the non-survivor group, with a gradual increase of difference between two groups. After adjusting for confounders, the mean daily increase was −6.014 (95% CI −7.076–4.953, P-value < 0.001). Baseline platelet demonstrated a U-shaped dose–response relationship with adverse outcomes in critical patients with AHF. Early elevation of platelet was correlated with higher in-hospital mortality, indicating that tracking early changes in platelet might help determine the short-term prognosis of critical patients with AHF.
Similar content being viewed by others
Introduction
Heart failure (HF) affects about 26 million people worldwide, with an overall prevalence of approximately 1.5–4.0% and increasing, which place enormous pressure on healthcare systems1,2,3,4. Acute heart failure (AHF) is a syndrome defined as the new onset or worsening of symptoms and signs of HF, mostly related to high mortality5. Hence, accurate assessment of severity in AHF patients have very important significance of improve prognosis and reduce medical burden. However, the complexity and heterogeneity of pathophysiology contribute to the severity of clinical symptoms in AHF patients ranging from mild to lethal6. Therefore, accurate assessment of disease severity in AHF patients has become a challenge.
Platelets (PLT), a component of blood, are involved in coagulation, the immune response and inflammation, and it has been proven to be closely related to the prognosis of critically ill patients7,8. Wang et al. study found that as a strong predictor of potential risk of death of critical patient with sepsis, every 10 × 109/L increase in platelets was associated with a 13% decrease in risk of the death9. In addition, the platelet to lymphocyte ratio (PLR) may be useful in supplementary assessment of short-term mortality risk in AHF patients10. However, few studies have explored the relationship between PLT and in-hospital death among critical patients with AHF. Furthermore, dynamical PLT change is a complementary predictor to baseline PLT for critical patient survival, even in patients without thrombocytopenia11. Therefore, dynamic assessment of platelet changes may be more conducive to an effective assessment of the overall condition and severity of AHF patients.
In the present study, we analyzed data from the medical information mart for intensive care III (the MIMIC-III) database, and to further revealed relationship of both baseline PLT and early changes in PLT within the first week after admission on in-hospital mortality among critical AHF patients.
Materials and methods
Source of data
All data of this study were obtained from the Medical Information Mart for Intensive Care III (MIMIC-III) database (version 1.4). The MIMIC-III database records medical data on patients in the intensive care unit (ICU) at Beth Israel Deaconess Medical Center between 2001 and 2012, with a collection of 53,423 ICU admissions, including a large amount of physiological data and medical records12. All data are authentic and publicly accessible for free. No informed consent was required from the patients as their basic personal information was anonymized (https://mimic.physionet.org/). Data for the present study was extracted by one author, Tao Liu, passed the training test and obtained permission to download and use the database (ID: 9008147).
study population
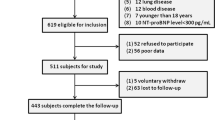
We initially recruited 3614 critical patients with the diagnosis of AHF from the MIMIC-III database, of which 607 participants were excluded due to repeated hospitalization and 42 participants were excluded due to death within 3 days of admission. Of the remaining 2,965 participants, 35 participants were excluded due to missing data. Ultimately, 2930 critical patients with AHF were identified for analysis, including 2720 survivors and 210 non-survivors.
Data extraction
The involved data was extracted with PostgreSQL tool. Demographic information: gender, age, ethnicity, and marital status; Vital signs: heart rate (HR), respiratory rate (RR), systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse oxygen saturation (SpO2), and temperature; 24 h urine output; Indicators of laboratory tests: platelet, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), potential of hydrogen (pH), N-Terminal pro-brain natriuretic peptide (NT-proBNP), white blood cell (WBC), neutrophile (N), lymphocyte (L), C reactive protein (CRP), and estimated glomerular filtration rate (eGFR); Comorbidities: sepsis, stroke, pneumonia, acute myocardial infarction (AMI), atrial fibrillation (AF), liver disease, chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), respiratory failure, and cancer; Therapeutic measures: renal replacement therapy (RRT), ventilation, and vasoactive drugs; Scoring scales for ICU disease: the Sequential Organ Failure Assessment (SOFA) Score and the Simplified Acute Physiology Score II (SAPS II). Length of hospital: length of ICU stay and length of hospital stay. All of the above baseline values were the first measured within the first 24-h after admission to the ICU. In addition, the registration information of admission and discharge was recorded, including the length of hospitalization, time of death, and length of stay in the ICU.
Outcomes and main exposure variables
The outcome of this study was in-hospital mortality, which was death from all causes during the patient's hospitalization. The independent variables were baseline platelet count and dynamically changed platelet count. Dynamically changed platelet counts were platelet counts measured daily during the one week stay in the ICU. The interval time between repeated measurements of platelet counts was irregular.
Statistical analysis
All data were analyzed with R Studio software (Version 4.2.1). Continuous variables that conformed to normal distribution were expressed as mean (standard deviation) and the t-tests were applied; Continuous variables not satisfying normal distribution were shown as median (interquartile spacing) [M (Q1, Q3)] and the Wilcoxon rank-sum tests were carried out; Categorical variables were presented as percentages (%) and the X2 tests were conducted. Sequentially, logistic regression models were constructed to assess the association of baseline platelet count and baseline tertile platelet count with in-hospital mortality in critical patients with AHF, and the results were displayed in the form of odds ratios (ORs) and 95% confidence intervals (CIs). Model 1: unadjusted variables; Model 2: Adjusted for age, gender, ethnicity, and marital status; Model 3: Adjusted for age, ethnicity, HR, RR, SBP, DBP, temperature, PO2, PCO2, pH, 24-h urine output, sepsis, AMI, AF, respiratory failure, RRT, ventilation, vasoactive drugs, SOFA, SASP II, NT-proBNP, WBC, N, L, CRP, eGFR, length of ICU stay, and length of hospital stay. Meanwhile, the relationship between baseline platelet counts and in-hospital mortality was re-analyzed utilizing stepwise regression to avoid multicollinearity among the covariates (Supplementary Table S1). The nonlinear relationship between baseline platelet count levels and in-hospital mortality were explored by adjusting the multivariate restricted cubic spline (RCS) regression model. The inflection point was calculated based on the recursive algorithm, and then the threshold effect analyses were conducted by the segmented logistic regression model (Supplementary Table S3). In addition, we conducted ROC for PLT, eGFR, and NT-proBNP on predicting in-hospital mortality, and we also examine correlation analysis of in-hospital mortality with WBC, CRP, neutrophil, lymphocyte, and PLT.
Differences in dynamical platelet counts between survivors and non-survivors during the first week after ICU admission were compared (Supplementary Table S4). Finally, generalized additive mixed models (GAMM) were constructed to investigate the relationship between dynamic platelet counts in the first week and in-hospital mortality in critical patients with AHF admitted to ICU. GAMM is usually performed to the analyse data obtained repeatedly, especially when the data have missing values or are duplicated irregularly13,14. P-value < 0.05 was considered to be statistically significant.
Ethics statement
Data of the present study was from the MIMIC-III database. The MIMIC III database was approved to build by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). Data for the present study was extracted by one author, Tao Liu, who has completed the online training course and passed the exam (ID: 9008147).
Results
Baseline characteristics of the study population
A total of 2930 critical patients with AHF were included with a mean age of 72.83 (13.14) years, of which 1486 (50.7%) were males and 1444 (49.3%) were females. After tracking hospitalization information, 210 (7.17%) deaths were recorded. Compared with the non-survivor group, patients in the survivor group were younger, had a higher proportion of Whites and Blacks, higher levels of temperature, DBP, SBP, PO2, PCO2, pH, eGFR, and platelets, lower levels of HR, RR, SAPSS II scores, and SOFA scores, and NT-proBNP, WBC, L, N, CRP, length of ICU stay, length of hospital stay, and lower prevalences of sepsis, pneumonia, AMI, AF, and respiratory failure, as well as lower proportions of ventilation, RRT and vasoactive medication use (all P-value < 0.05). There were no statistically significant differences between the two groups in terms of the proportion of gender and marital status, levels of SPO2, prevalence of stroke, COPD, cancer, hypertension, diabetes and CKD (all P-value > 0.05) (Table 1). In addition, the results of propensity score matching (1:3) was replaced in Supplementary Table S2.
Association between in-hospital mortality and baseline platelet
The detailed results of the multivariate logistic regression are shown in Table 2. When considering platelets as a continuous variable, each 1 × 109/L increase in platelet count corresponded to a OR (95% Cl) for in-hospital mortality of 0.997 (95% CI 0.994–0.999, P-value = 0.018). Similar results were found when stepwise regression regression analysis was performed (Supplementary Table S1. Baseline platelets were considered as a tertile variable, and the fully adjusted OR (95% CI) for in-hospital mortality was 0.548 (0.328–0.917, P-value = 0.022) and 0.712 (0.431–1.176, P-value = 0.185) for the Q2 and Q3 groups, respectively, on the basis of Group Q1 as a reference.
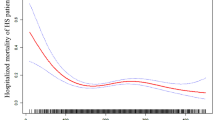
The non-linear relationship
RCS regression model adjusted for model 3 showed that the relationship between baseline platelets and in-hospital mortality was U-shaped in critical patients with AHF (Fig. 1). The results of two piecewise linear regression revealed that the in-hospital mortality was lowest when the baseline platelet count was 209 × 109/L (Supplementary Table S3). Below the inflection point, in-hospital mortality declined with increasing platelet counts [OR (95% CI): 0.990 (0.985–0.995), P-value < 0.001]. Above the inflection point, in-hospital mortality rose with increasing platelet counts [OR (95% CI): 1.002 (0.998–1.006), P-value = 0.380]. The C-statistics of platelet count were calculated. PLT, e-GFR and NT-proBNP all have certain clinical predictive value in predicting in-hospital death in patients with AHF, and PLT could improve predictive value of e-GFR and NT-proBNP in predicting in-hospital death (Supplementary Table S5). Meanwhile, we also analyzed correlation of between PLT, WBC, CRP, neutrophil, lymphocyte and hospital mortality. PLT are negatively related hospital mortality, and positively related to WBC, neutrophil. WBC, neutrophil, and lymphocyte are positively related to hospital mortality (Supplementary Figure S1).
The RCS for association between baseline platelet counts and in-hospital mortality with adjusting for adjusted for age, ethnicity, HR, RR, SBP, DBP, temperature, PaO2, PaCO2, pH, 24-h urine output, AMI, AF, respiratory failure, RRT, ventilation, vasoactive drugs, SOFA, SASP II, NT-proBNP, WBC, N, L, CRP, eGFR, length of ICU stay, and length of hospital stay.
Association between in-hospital mortality and dynamically changed platelets
We compared the dynamics of platelet counts at 4 time points: 1st, 2nd-3rd, 4th-5th, and 6th-7th days after ICU admission between the survivor and non-survivor groups (Supplementary Table S3). The difference in platelet counts between the two groups at each time point was statistically significant. In the next step, the trajectories of platelet count over time for two groups were plotted with the GAMM model fully adjusted for confounding factors (Fig. 2). Platelet counts of patients in the survivor group showed a decreasing and then increasing trend with a U-shape. The platelet counts in the non-survivor group decreased gradually within 1 week after admission to ICU. Overall, the difference in platelet counts between the two groups tended to increase over time, and platelet counts were consistently higher in the survivor group than in the non-survivor group. Platelet counts increased by a mean of −5.989 per day in the non-survivor group compared to the survivor group. Consistent results were also observed in Model 3 (β = −6.014, 95% CI −7.076–4.953, P-value < 0.001) (Table 3).
Evaluation of association between platelet counts (1–7 days) and in-hospital mortality in critical patients with AHF using generalized additive mix model (GAMM), and adjusting for adjusted for age, ethnicity, HR, RR, SBP, DBP, temperature, PaO2, PaCO2, pH, 24-h urine output, AMI, AF, respiratory failure, RRT, ventilation, vasoactive drugs, SOFA, SASP II, NT-proBNP, WBC, N, L, CRP, eGFR, length of ICU stay, and length of hospital stay.
Discussion
The present study observed the dynamic evolution of platelet counts in critical patients with AHF from the 1st to the 7th day after ICU admission in order to analyze the relationship between platelet counts and poor outcomes. A U-shaped association between baseline platelet count and in-hospital mortality was found. Platelet counts of patients in the survivor group presented a first decreasing and then increasing trend on days 1–7 after admission to the ICU, while the non-survivor group showed a continuous decreasing trend, and platelet counts in the survivor group were consistently higher than those in the non-survival group at the same time points. After sufficient adjustment for confounders, we found that the early decline in platelet counts was an independent predictor of in-hospital death in critical patients with AHF.
The association of decreased platelet count with poor prognosis has been well supported in studies among different populations, especially in ICU patients15,16,17,18. Nihat Polat et al. revealed that in patients with acute decompensated HF, baseline platelet counts were significantly higher in alive patients than in deceased patients, and lower platelet counts predicted an increased risk of death at one year19. Results from the MyoVasc study showed that lower platelet counts were associated with poorer cardiac function and were an independent predictor of worsening heart failure, with a median follow-up of 2.24 years20. The indicators in all three studies were platelet counts measured at a single time point at baseline and do not take into account the fact that platelet counts change dynamically in actual clinical management. Current studies on the relationship between platelet dynamic trajectories and prognosis have focused on severe burn patients, septic shock patients, and cancer patients, and studies involving critical patients with AHF have not been observed21,22,23. We investigated the relationship between early changes in platelet counts and the risk of in-hospital mortality in critical patients with AHF, which more rationally assesses the evolution of the disease holistically.
Univariate analysis of this study indicated that baseline platelet counts were higher in the alive group than in the dead group, which is consistent with previous studies19,24. The results of the multivariate logistic regression also endorsed these findings, regardless of considering platelets as a continuous variable or a tertile variable. Immediately after, GAMM was implemented to explore early changes in platelet counts in critically ill AHF patients and their relationship to the risk of in-hospital mortality. The results showed that the platelet counts of those who died were lower than those of the survivors on the 1st-7th days and showed a continuous decline, with a statistically significant difference between the groups. In summary, we need to focus on both the platelet count at baseline and the dynamic changes in platelet count on the patient's prognosis.
AHF is a clinical syndrome characterized by the rapid onset or sudden deterioration of symptoms or signs of HF with elevated plasma natriuretic peptide levels caused by multiple etiologies25. It is well known that inflammation plays an important role in the pathogenesis of HF, and platelets are the "instigators" and "'participants" of inflammation26. During the inflammatory response, vascular endothelial cells are damaged, followed by platelet adhesion, aggregation, and thrombus formation27. In addition, platelet activation promotes the inflammatory response and the process of atherosclerosis by initiating monocyte transformation, recruiting inflammatory cells, and promoting foam cell formation28. On the other hand, platelet activation also releases mediators such as serotonin, adenine nucleotides, and other mediators thereby activating protective cardiac pathways29,30. It has been reported that injecting autologous platelet gel into the myocardium of mice can improve cardiac function and delay ventricular remodeling after myocardial infarction31. Therefore, platelets have both protective and damaging effects on the heart. Platelets in patients with heart failure are usually overactivated, which may be attributed to hemodynamic changes and structural damage to the vascular system29,32,33. Platelet overconsumption and high destruction lead to a decrease in peripheral blood platelet counts. In addition, low platelet count is related to activation of the renin–angiotensin–aldosterone system 34. Therefore, the poorer prognosis among critical patients AHF with low platelet count might appear. Moreover, platelets play a role in the anti-infectious response35. Previous studies have shown that when the daily PLT count increases by 31 × 109/L in ICU patients with baseline PLT counts exceeding 300 × 109/L, the risk of death in ICU patients is reduced by 25%11. In addiion, the daily PLT count increases by 10 × 109/L in ICU patients with baseline PLT counts below 100 × 109/L, the risk of death in ICU patients with sepsis is reduced by 13%9. Therefore, a certain increase in PLT count leads to better prognosis, which is consistent with our findings. In the present study, we found a U-shaped dose–response association of between baseline PLT counts and in-hospital mortality in critical patients with AHF.
There are some limitations in this study: (1) Although the confounding factors were adequately adjusted, it was not possible to control all the confounding parameters that could affect the results. (2) The retrospective study design, which could only show the correlation between PLT counts and in-hospital mortality, did not prove causality. (3) This is a single-center study, and the results are representative of the study population, with some limitations on extrapolation. Therefore, further multi-center registries of large-scale prospective studies are needed to confirm. (4) This study did not conduct further subgroup analysis on the causes of heart failure. Later studies need to conduct subgroup analysis on the causes of heart failure to verify the stability of the relationship between PLT and in-hospital mortality. (5) This article focuses on correlations, and more research is needed to uncover the exact mechanism of the association between PLT counts and patients with AHF.
Conclusions
There was a U-shaped relationship between baseline PLT counts and in-hospital mortality in critical patients with AHF. Early elevation of PLT was correlated with higher in-hospital mortality, indicating that tracking early changes in PLT might help determine the short-term prognosis of critical patients with AHF. In addition, PLT count provides certain clinical predictive value for the short-term prognosis of critical patients with AHF. Future research should focus on investigating the association between PLT counts and long-term outcomes.
Data availability
The data supporting the findings of the present paper could be found at https://mimic.mit.edu/.
References
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card Fail Rev 3, 7–11 (2017).
Dharmarajan, K. et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA 318, 270–278 (2017).
Lainščak, M. et al. Sex- and age-related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long-Term Registry. Eur J Heart Fail 22, 92–102 (2020).
Li, L. et al. Assessing the evidence-practice gap for heart failure in China: the Heart Failure Registry of Patient Outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail 22, 646–660 (2020).
Arrigo, M. et al. Acute heart failure. Nat Rev Dis Primers 6, 16 (2020).
Joseph, S. M., Cedars, A. M., Ewald, G. A., Geltman, E. M. & Mann, D. L. Acute decompensated heart failure: contemporary medical management. Tex Heart Inst J 36, 510–520 (2009).
Middleton, E. A., Weyrich, A. S. & Zimmerman, G. A. Platelets in pulmonary immune responses and inflammatory lung diseases. Physiol Rev 96, 1211–1259 (2016).
Brummel, N. E. et al. Inflammation and coagulation during critical illness and long-term cognitive impairment and disability. Am J Respir Crit Care Med 203, 699–706 (2021).
Wang, D., Wang, S., Wu, H., Gao, J., Huang, K., Xu, D. & Ru, H. Association between platelet levels and 28-day mortality in patients with sepsis: a retrospective analysis of a large clinical database MIMIC-IV. Front. Med. (Lausanne) 9, 833996 (2022).
Turcato, G. et al. Evaluation of neutrophil-lymphocyte and platelet-lymphocyte ratios as predictors of 30-day mortality in patients hospitalized for an episode of acute decompensated heart failure. J Med Biochem 38, 452–460 (2019).
Chen, J. et al. Association of longitudinal platelet count trajectory with ICU mortality: A multi-cohort study. Front Immunol 13, 936662 (2022).
Johnson, A. E. et al. MIMIC-III, a freely accessible critical care database. Sci Data 3, 160035 (2016).
Najjar, S. S. et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 51, 1377–1383 (2008).
Zhao, X. et al. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients-indications for predictive, preventive, and personalized medical approach. EPMA J 11, 139–145 (2020).
Puertas, M., Zayas-Castro, J. L. & Fabri, P. J. Statistical and prognostic analysis of dynamic changes of platelet count in ICU patients. Physiol Meas 36, 939–953 (2015).
Chen, Y. et al. Relationship of platelet counts and inflammatory markers to 30-day mortality risk in patients with acute type A aortic dissection. Biomed Res Int 2020, 1057496 (2020).
Schupp, T. et al. Diagnostic and prognostic role of platelets in patients with sepsis and septic shock. Platelets 34, 2131753 (2023).
Zhou, Y. et al. Enhanced red blood cell distribution width to platelet ratio is a predictor of mortality in patients with sepsis: a propensity score matching analysis based on the MIMIC-IV database. BMJ Open 12, e062245 (2022).
Polat, N. et al. The importance of hematologic indices in the risk stratification of patients with acute decompensated systolic heart failure. Turk Kardiyol Dern Ars 43, 157–165 (2015).
Dahlen, B. et al. The impact of platelet indices on clinical outcome in heart failure: Results from the MyoVasc study. ESC Heart Fail 8, 2991–3001 (2021).
Gao, Y., Mei, Q. & Pan, A. Dynamic changes of platelet count in first week and their predictive value for prognosis in septic shock patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 34, 1055–1059 (2022).
Huang, X. et al. Relation between dynamic changes of platelet counts and 30-day mortality in severely burned patients. Platelets 30, 158–163 (2019).
Yang, W. et al. Dynamic changes of platelets before and after surgery predict the prognosis of patients with operable non-small cell lung cancer. J Cancer 13, 823–830 (2022).
Mojadidi, M. K. et al. Thrombocytopaenia as a prognostic indicator in heart failure with reduced ejection fraction. Heart Lung Circ 25, 568–575 (2016).
McDonagh, T. A. et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart. Fail. 24, 4–131 (2022).
Chung, I. & Lip, G. Y. Platelets and heart failure. Eur Heart J 27, 2623–2631 (2006).
Sharma, S., Tyagi, T. & Antoniak, S. Platelet in thrombo-inflammation: Unraveling new therapeutic targets. Front Immunol 13, 1039843 (2022).
Bakogiannis, C., Sachse, M., Stamatelopoulos, K. & Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 122, 154157 (2019).
Ziegler, M., Wang, X. & Peter, K. Platelets in cardiac ischaemia/reperfusion injury: A promising therapeutic target. Cardiovasc Res 115, 1178–1188 (2019).
Malik, A. et al. Exogenous SDF-1α protects human myocardium from hypoxia-reoxygenation injury via CXCR4. Cardiovasc Drugs Ther 29, 589–592 (2015).
Cheng, K. et al. Intramyocardial injection of platelet gel promotes endogenous repair and augments cardiac function in rats with myocardial infarction. J Am Coll Cardiol 59, 256–264 (2012).
Kim, J. H., Shah, P., Tantry, U. S. & Gurbel, P. A. Coagulation abnormalities in heart failure: Pathophysiology and therapeutic implications. Curr Heart Fail Rep 13, 319–328 (2016).
Jafri, S. M. et al. Platelet function, thrombin and fibrinolytic activity in patients with heart failure. Eur Heart J 14, 205–212 (1993).
Schäfer, A. et al. Inhibition of platelet activation in congestive heart failure by aldosterone receptor antagonism and ACE inhibition. Thromb Haemost 89, 1024–1030 (2003).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 13, 463–469 (2007).
Funding
The Shanghai Jinshan District Health Commission Project Fund (grant number: JSKJ-KTQN-2022-11 and JSKJ-KTMS-2020-09), the Shanghai Jinshan District Medical and Health Science and Technology Innovation Fund Project (grant number: 2022-WS-61), and Shanghai University of Medical & Health Sciences Research Fund Project (Grant Number: SSF-23-25-002) supported the present study.
Author information
Authors and Affiliations
Contributions
W.L.L designed the study and drafted the manuscript. L.T, Z.J.Z and W.B analyzed the data. Z.G.L and P.Y.S critically revised the manuscript. S.L.F supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, L., Liu, T., Zhu, Z. et al. Associations between static and dynamic changes of platelet counts and in-hospital mortality in critical patients with acute heart failure. Sci Rep 14, 9147 (2024). https://doi.org/10.1038/s41598-024-59892-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59892-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.