Abstract
The development of novel antioxidant compounds with high efficacy and low toxicity is of utmost importance in the medicine and food industries. Moreover, with increasing concerns about the safety of synthetic components, scientists are beginning to search for natural sources of antioxidants, especially essential oils (EOs). The combination of EOs may produce a higher scavenging profile than a single oil due to better chemical diversity in the mixture. Therefore, this exploratory study aims to assess the antioxidant activity of three EOs extracted from Cymbopogon flexuosus, Carum carvi, and Acorus calamus in individual and combined forms using the augmented-simplex design methodology. The in vitro antioxidant assays were performed using DPPH and ABTS radical scavenging approaches. The results of the Chromatography Gas-Mass spectrometry (CG-MS) characterization showed that citral (29.62%) and niral (27.32%) are the main components for C. flexuosus, while d-carvone (62.09%) and d-limonene (29.58%) are the most dominant substances in C. carvi. By contrast, β-asarone (69.11%) was identified as the principal component of A. calamus (30.2%). The individual EO exhibits variable scavenging activities against ABTS and DPPH radicals. These effects were enhanced through the mixture of the three EOs. The optimal antioxidant formulation consisted of 20% C. flexuosus, 53% C. carvi, and 27% A. calamus for DPPHIC50. Whereas 17% C. flexuosus, 43% C. carvi, and 40% A. calamus is the best combination leading to the highest scavenging activity against ABTS radical. These findings suggest a new research avenue for EOs combinations to be developed as novel natural formulations useful in food and biopharmaceutical products.
Similar content being viewed by others
Introduction
In the last two decades, special interest has been dedicated to exploring novel, effective, and safe antioxidants from natural products to avoid oxidative damage of living cells induced by reactive chemical species (RCS). Free radicals represent the major RCS implicated in the oxidation process. They contain an unpaired electron on the valence orbital and are highly reactive1,2. Free radicals such as hydroxyl radical (HO·), hydrogen peroxide (H2O2), superoxide anion (O2·−), oxygen singlet (1O2), peroxynitrite (NO3−) and nitric oxide (·NO) radical may cause the alteration of vital macromolecules, including lipids, DNA, and proteins3,4. This event leads to cell injury and disruption of normal homeostasis5, and thus trigger a number of pathological disorders, such as cancer and inflammatory diseases6,7. Therefore, the use of external sources of antioxidants can help maintain a certain equilibrium between free radicals and the antioxidant system and thus preserve physiological functions. However, the available commercialized antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) have recently been demonstrated to be hazardous for human health1,8,9. In fact, researchers have recently turned their attention to new preservatives, such as the development of new and non-toxic antioxidant formulations from natural resources, especially essential oils (EOs). EOs are defined as multifaceted bioactive components that comprise various classes of chemical compounds, including monoterpenes, alcohols, sesquiterpenes, aldehydes, phenols, esters, ethers, ketones10,11,12,13. They possess important antioxidant properties and are found applications as natural antioxidants in the food industry and pharmacy14,15,16.
Cymbopogon flexuosus is commonly known as lemongrass and belongs to the Poaceae family, which is also known as the grass family. C. flexuosus has shown several traditional uses and is known for its therapeutic properties, including antimicrobial, anti-inflammatory and anticancer propertie17. C. flexuosus is widely used in cooking to add a citrusy and aromatic flavor to dishes. It is often used in soups, curries, marinades, and teas18.
Carum carvi, commonly known as caraway and belonging to the Apiaceae family, is present in natural vegetation and is also deliberately grown as both a medicinal herb and a culinary plant in various regions around the world19. C. carvi has been traditionally used for various medicinal purposes. It has shown carminative properties, helping to alleviate digestive problems such as indigestion, bloating, and flatulence. C. carvi has also been used to relieve spasms and cramps in the digestive system and could contribute to treating inflammation and respiratory conditions20.
Acorus calamus, commonly known as sweet flag or calamus, is a perennial herbaceous plant that has been utilized for various medicinal and common purposes21. Belonging to the Acoraceae family, this herbaceous plant has a long history of traditional use in different cultures around the world. Calamus has a range of potential medicinal uses. Traditionally, it has been used as a remedy for digestive ailments. The rhizomes of A. calamus are believed to possess carminative properties, aiding in the relief of indigestion, bloating, and gas22. Moreover, it has been used as an expectorant, helping to ease respiratory congestion and coughs. Some traditional medicinal systems also suggest its use for mental clarity and memory enhancement. Besides its traditional applications, A. calamus has been employed in various other common uses. One notable use is as a fragrant herb in the perfume and cosmetics industries. The aromatic qualities of the plant make it a popular choice for adding fragrance to products23.
Interestingly, recent reports have demonstrated that the use of combined EOs can enhance their antioxidant effect24,25,26. While the synergistic interactions between EOs are yet fully understood, such knowledge, particularly regarding the proportions and interactions mechanisms between each component in a mixture, is important in order to discover novel and effective combinations. With this in mind, we designed a new conception of the mixture to assess the antioxidant ability generated through the interactions of three oils from Cymbopogon flexuosus, Carum carvi, Acorus calamus using an augmented-simplex design methodology. This method provides the necessary EOs concentrations that exhibit synergistic antioxidant effect.
Materiel and methods
Plant material and extraction of EOs
The plants Acorus calamus L. (aerial parts), Cymbopogon flexuosus L. (aerial parts), and Carum carvi L. (seeds) were harvested from a farmer (Mernissa) in the Taounate region (34° 39′ 03″ N, 4° 16′ 40″ W), North of Morocco. The plants identification was performed at the Scientific Institute, Mohammed V University in Rabat, by Pr. Mohamed Ibn Tattou under voucher codes RAB 11,415–11,417. The current study conformed to all applicable institutional, national, and international guidelines and regulations. The samples were dried in appropriate conditions (continuous ventilation at dark place) and then extraction of EOs was undertaken. In fact, an amount of 100 g of dried plants was subject to hydro-distillation for 180 min using Clevenger-type device. The distilled oils were recuperated and dehydrated by anhydrous sodium sulfate, filtered, and kept at 4 °C, pending upcoming tests.
GC–MS analysis
The chemical characterization of the three EOs was performed using chromatography (GC) (Trace GC-Ultra) attached with mass spectrometry (MS) (Quadrapole, Polaris Q) (GC–MS) as described by Benkhaira et al.27. The machine is equipped with a non-polar HP-5MS capillary column (30 m, 0.32 mm × 0.25 µm). The temperature of the injector and detector was set at 280 and 300 °C, respectively. The column temperature was monitored at 50 °C for 5 min and then at 180 °C for 4 °C/min and. Helium (He) was used as the carrier gas (1.3 mL/min). A volume of 0.5 µL of EOs was manually injected. The chemical identification of samples components was established based on the comparison of their respective retention index (RI) (obtained and calculated based on homologous series of alkanes ranging from C8 to C24) and mass spectral (MS) fragmentation patterns with those recorded in the published articles28,29. Moreover, the individual constituents were quantified through internal normalization of the total area of peaks noticed in each chromatogram. The MS of each constituent was completed according to the data stored in chemical libraries (NIST/EPA/NIH MASS SPECTRAL LIBRARY Version 2.0, July 1, 2002) via computer matching. To determine the relative peak area percentage of a mixture, such as an essential oil (EO), the initial step involves the summation of all individual peak areas. Then, to calculate the percentage of each EO component, we divide its individual area by the total area and multiply the result by 100.
Antioxidant assays
DPPH radical scavenging assay
2,2-diphenyl 1-picrylhydrazyle (DPPH) was used to determine the antiradical activity of three EOs as well as their different combinations generated by experimental design approach. A modified version of protocol by El Hachlafi et al.30 was adopted for this subject. Specifically, 200 mL aliquots samples of different concentrations previously prepared in methanol were added to 1.4 mL of DPPH solution (0.004%). The obtained solutions were then incubated for 35 min at dark place. Afterwards, the optical density was established at 517 nm using a spectrophotometer. Butylated hydroxytoluene (BHT) was used as a reference.
The scavenging ability of the EOs was estimated using Eq. (1):
where ODC and ODEO is the optical density of the control and tested EOs, respectively.
ABTS radical scavenging activity
2,2′-azino-bis 3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical scavenging method was performed as indicated by Al-Mijalli et al.10, with minor modifications. Briefly, the cationic ABTS radical was produced by reacting 10 mL of the solution of ABTS (2 mM) with 0.1 mL of potassium persulfate solution (70 mM). The obtained combination was kept in a dark place for 14 h. Then, the mixture was diluted with methanol until an optical density of 0.700 ± 0.02 at 734 nm was obtained. A volume of 0.2 mL of samples at different concentrations was mixed with 2 mL of the diluted ABTS solution. After incubation for 2 min, the optical density was determined against a blank (methanol) at 734 nm. ABTS scavenging potential was represented as IC50 (μg/mL) ± SD (n = 3). Butylated hydroxytoluene (BHT) was used as a reference drug.
Experimental design
Mixture design
An augmented simplex-centroid design technique was adopted to identify the optimum antioxidant effect of the combination of EOs of C. flexuosus, C. carvi, A. calamus as described by Benkhaira et al.27. The EO system constituents are listed in Table 1. Each EOs may possess a value ranging between 0 and 1 in the mixture, and the sum of three components is equal to 1 (Table 1). In addition, the DPPHIC50 and ABTSIC50 responses were used to assess the antioxidant ability of the studied EOs.
Experimental matrix and mathematical model
In the current investigation, a total of 10 trials were performed and are depicted as an equilateral triangle (Fig. 1), comprising three pure components (1), corresponding–to triangle apexes (X1, X2, X3), binary combination (0.5/0.5) marked by midpoints of the triangle (X4, X5, X6), equal proportional of the three components (0.33/0.33/0.33), detected at the gravity center of the triangle (X7). This trial was carried out in three separate replicates, and finally the three control points (X10, X11, X12), comprising ternary mixtures (0.67/0.16/0.16). A cubic model was used to indicate responses according to the independent variable using the formula below (2):
where Y is the experimental response established by IC50 (µg/mL); Ω1, Ω2, and Ω3 are linear regression coefficients, Ω12, Ω13 and Ω23 are binary regression coefficients, Ω123 is the ternary regression coefficient, and ɛ is the regression error term.
Statistical analysis
The ration Fratio (MSR/MSr) between the mean square regression and mean square residual was used to assess the statistical significance of the mathematical model at a 95% confidence level31. A greater F-value justifies the variation of the results around their mean. Furthermore, the ratio of the mean square Lack of fit (MSLOF) and mean square pure error (MSPE) FratioLOF/PE were employed to examine if the postulated mathematical model was fitted with the observed results. High F ratioLOF/PE values indicate incorrectness of the model32. Besides, the coefficient of determination (R2) was computed to express the quality of the presumed mathematical models. The t-student test was applied to evaluate the importance of the estimated factors33. Moreover, the F-test for ANOVA analysis was conducted to verify the significance of the assumed models. The experimental design approach and the graphical and statistical analysis were performed using Design Expert software version 12 and SAS JMP® version 14. The results were established as the means ± SD (n = 3).
Optimization tools
The contour plot and 3D surface for the isoresponse curves were employed to reveal the compromise areas between studied components34. Besides, the desirability tool was applied to promote the precise optimal value with a perfect percentage of compromise. This function ensures the maximal adjustment mathematical model, which ranges between 0 and 1. A value of 0 indicates an undesirable response, while a value of 1 signifies a highly desirable response35.
Results and discussions
Chemical profile of the three EOs
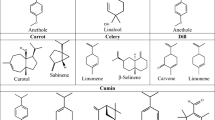
The chemical profile, molecular formula, percentage, and yield of each three essential oils are displayed in Table 2. The yield of the EO extracts from A. calamus, C. carvi and C. flexuosus arew 0.9, 1.1 and 2.3 (v/w). Each of them contains eighteen, nine and thirty phytoconstituents which account for 98.07, 99.44 and 97.61% of the corresponding plants. β-Asarone (69.11%) is the most abundant compound in A. calamus EO, followed by oxygenated sesquiterpenes (6.17%), sesquiterpene hydrocarbons (4.31%) and asaraldehyde (1.34%) as the single monoterpene (oxygenated).
The chemical profile of A. calamus EO is consistent with other earlier studies conducted in the Polish region, where Szczeblewski et al.36 found that asarones were among the most prevalent components. Similarly, Loying et al.15 revealed that β-asarone is the major compound (82.42%) along with other minor compounds such as calaraene (2.41%) and euasarone (1.92%) in the North-East India region. Generally, β-asarone was found in high amount in the East Asia, following by the European areas37.
Of the nine compounds detected in C. carvi, about 69.86% of the constituents comprise oxygenated monoterpenes, including d-carvone (62.09%) along with other minor compounds such as p-menth-8-en-2-one (3.01%), cis-carveol (1.28%) and limonene-1,2-diol (1.24%), as well as d-limonene (29.58%). These results are consistent with another study by Ghannay et al.38, in which carvone (58.2%) and limonene (38.5%) are the main detected compounds. Similarly, Lasram et al.39 reported that C. carvi EOs contained 15 different oxygenated and hydrocarbons monoterpenes which are dominated by carvone (78.85%) and limonene (18.62%).
As for C. flexuosus, a total of thirty compounds have been detected. This extract is predominantly composed of oxygenated monoterpenes (67.45%), followed by citral (29.62%) niral (27.32%), and sesquiterpene hydrocarbons (9.6%), whereas minor constituent was detected for monoterpene hydrocarbons (6.58%). The result is consistent with a previous studies where citral and niral were reported to be the major components with a composition of 29.4% and 30.4% respectively40. In an investigation of the effect of altitude on chemical profile by Pathania et al.41, citral was found as the main compound with a composition ranging from 13.61% to16.14%. Apart from altitude, it was reported that the presence and quantity of chemical constituents may vary due to the intervention of various characteristics, such as climate (temperature, humidity), soil (pH, N:P ratio, mineral) and plant growth cycle35.
This reinforces the assertion proposing that ecological, climatic, and nutritional conditions quantitatively and qualitatively affect EO components in plants. Several studies have clarified the positive connection between variations in external and internal factors of plants, including climate, seasonal variation, soil composition, metabolic pathways, and the chemical constituents of EOs10,42.
Antioxidant activity
In the current investigation, two methods were used to assess the antioxidant activity of the EOs extract from C. flexuosus, C. carvi, and A. calamus, namely the DPPH and ABTS radicals scavenging assays. As shown in Fig. 2, the three sources of EOs demonstrated variable antiradical activity on DPPH and ABTS radicals. C. carvi possesses high antioxidant activity on both ABTS and DPPH tests with an IC50 value of 152.67 ± 3.19 µg/mL and 194.1 ± 2.01 µg/mL, respectively, while A. calamus exhibits relatively weaker scavenging activity with an IC50 = 193.42 ± 2.52 µg/mL and 349.04 ± 3.73 µg/mL in the corresponding antioxidant assays. The antioxidant activity for C. flexuosus is the weakest, with IC50 values of 874.22 ± 4.13 µg/mL and 1034.17 ± 5.87 µg/mL for DPPH and ABTS radicals, respectively. The scavenging activity of the three EOs was found to be significantly different (p < 0.05) to the standard reference BHT that was used as a positive control (IC50 = 114.07 ± 1.42 µg/mL for ABTS and 191.13 ± 0.83 µg/mL for DPPH). The higher antioxidant activity of EOs under optimal conditions results from a combination of factors that support optimal plant growth, metabolism, and defense mechanisms43. These conditions create an environment where plants can produce and accumulate higher levels of antioxidant compounds in their essential oils. Additionally, EOs production and antioxidant activity are influenced by nutrient availability. In optimal conditions, plants have access to essential nutrients such as minerals and vitamins, which play a crucial role in the synthesis of antioxidants1. Optimal conditions can also influence the expression of genes related to EO constituents and antioxidant synthesis. Under favorable environmental conditions, the plant may upregulate genes implicated in the synthesis of specific antioxidant compounds44.
As of to date, there have been several investigations reported on the antioxidant potential of EOs from the three plants using different in vitro tests, including DPPH, ABTS, ORAC, FRAP, and β-carotene bleaching tests14,15,45,46,47,48.
EOs with the highest antiradical ability may play a pivotal role in diseases prevention, such as neurodegenerative pathologies, cancer, inflammatory disease, and immune system decline10. They could protect cell from the harmful effects generated by free radicals, thus preserving proper physiological function13,49. It is well known that volatile components possess significant antioxidant activity in different biological systems11,30. The antioxidant activity of the studied EOs could be ascribed to the presence of definite chemical classes, including alcohol, ether, ketone, aldehyde, monoterpene, and sesquiterpenes, which demonstrate high ability in neutralizing free radicals and disrupting peroxides50,51. In our study, the high scavenging activity of EOs extracted from C. carvi could also be due to the abundance of monoterpenes, such as carvone, which possesses a conjugated double bond in its structure and thereby induce antioxidant activities12,52. Additionally, carvone has been considered as a protective molecule against lipid peroxidation12. Furthermore, the monoterpenes citral, a major component identified in C. flexuosus EO, has also shown promising antioxidant properties53,54. Similar to monoterpenes, sesquiterpenes can act as direct antioxidants, neutralizing free radicals. Some sesquiterpenes may also modulate cellular signaling pathways related to oxidative stress and inflammation.
Simplex centroid design
The simplex-centroid design, comprising various mixtures of the three tested EO extracts (C. flexuosus, C. carvi and A. calamus) and the recorded response (DPPHIC50 and ABTSIC50) of each experiment, is summarized in Table 3. C. flexuosus, C. carvi and A. calamus essential oils are known for their potential health and wellness benefits. Investigating their combination can contribute to the development of products that relieve oxidative stress and other positive effects. Furthermore, to the best of our knowledge, there is no published work on the combination effect of C. flexuosus, C. carvi and A. calamus essential oils using experimental design methodology. Indeed, choosing to explore this combination is a commendable and innovative approach.
The trials (consisting of 12 experiments) were randomized, and each recorded response is the mean of three independent repetitions. The results revealed that the antioxidant activity tests for DPPHIC50 and ABTSIC50 range from 185.34 ± 1.07 to 874.22 ± 4.13 µg/mL and from 82.25 ± 1.06 to 1034.17 ± 5.87 µg/mL, respectively. Data analysis indicated that the equal-proportional combination of the three studied EO extracts was the best formulations exhibiting the highest scavenging potential on ABTS and DPPH radicals.
Statistical validation of postulated model
Variance analysis was conducted to investigate the interaction of various components in the combination, as illustrated in Table 4. The findings demonstrate that the main regression impact was statistically significant for both analyzed responses because the significance of the risk (p-value) is lower than 0.05 (0.0024 and 0.0007 for DPPHIC50 and ABTSIC50, respectively). Evidently, the F(R/r) determined for the two examined responses (19.98 for the DPPHIC50 response and 34.29 for the ABTSIC50 response) demonstrated that it is greater than the tabular value of F at the 95% confidence level. In addition, the ANOVA F-test demonstrated that both assumed models had no lack of fit, since their p-values were less than 0.05 (0.0123 and 0.0319). The calculated F Ratio (LOF/PE) of the studied responses was less than the theoretical value FLOF/PE (F 0.05; 3.2 = 19.16) at 95% of confidence.
The importance of a model increases with the coefficient of determination (R2) and the adjusted R2 values. As a result, according to Table 4, the coefficients of determination (R2) were 0.97 and 0.98 for DPPHIC50 and ABTSIC50, respectively, whereas the R2adj was equal to 0.88 and 0.95 for DPPHIC50 and ABTSIC50, respectively. These analyses reflect a good concordance between the expected and actual data of the assumed models. As a confirmation of these results, the graph (Fig. 3) illustrates a linear curve for the observed values as a function of the expected ones for both studied responses.
Compounds effects and adjusted models
Table 5 provides an overview of the special model's estimated regression coefficients. The regression equations with significant coefficients (p-values < 0.05) were employed to identify the correlations between all tested factors and the attained responses for DPPHIC50 and ABTSIC50.
The statistically significant coefficients for DPPHIC50 response are those indicating the effects of individual components (Ω1, Ω2, and Ω3), as well as the ternary interaction term Ω 123. However, the coefficients of the binary interaction terms (Ω12, Ω13, and Ω23) are nonsignificant (p > 0.05) and indicate no impact on DPPH radical. Indeed, after excluding all non-significant coefficients from the assumed models, the mathematical models describing the response as a function of the tested components are expressed by Eq. (3):
Concerning the ABTSIC50 response the significant terms were Ω1, Ω3 and Ω123. These outcomes affirm that the antioxidant potential on ABTS radical depends especially on the effect of C. flexuosus and A. calamus EOs as well as the ternary effect.
Thus, the adopted mathematical model is expressed by Eq. (4):
Optimization of formulation and desirability study
The optimization operation adopting the experimental design methodology consists of identification of the optimum modulation of the studied components proportions, resulting in the best response values. While the statistically confirmed mathematical models are expected, these optimal outcomes do not necessarily align with those reported in the 12 performed tests; however, they could predict them with high accuracy in the investigated experimental area55. In order to gain valuable insights into the best attainable values, we start with the better value when executing the tests56. Hence, the best documented results were 185.34 ± 1.07 and 82.25 ± 1.06 µg/mL for DPPHIC50 and ABTSIC50, respectively. As a result, a setting of the factors enabling the attainment of responses higher than or equal to these readings was accepted.
Mixture profile
The contour plot and 3D surface (2D and 3D mixture plots, respectively) in Fig. 4 illustrate the optimal combination of the three EO extracts, namely C. flexuosus, C. carvi and A. calamus that maximize both responses (DPPHIC50 and ABTSIC50). This graph demonstrated the relationship between the responses and the proportions of each antioxidant agent34,57. It is produced by the Design-Expert software based on iso-responses curves, which are perfectly suited for examining the ideal conditions to achieve the best response values. The blue color indicate lower IC50 values and higher antioxidant potential, whereas yellow to dark red color signifies moderate to higher IC50 values.
Optimization of DPPH IC50
As depicted in the 2D and 3D mixture plots (Fig. 4A), the optimal compromise area (dark blue zone) corresponding to the best DPPH-IC50 value, which equal to 185.34 μg/mL, requires the use of a ternary combination of C. flexuosus, C. carvi and A. calamus. These data are validated by the desirability test (Fig. 5A), in which the optimal value of the DPPH-IC50 response that could be reached is equal to 183.88 μg/mL with a compromise percentage of 99.9%. This outcome can be attained by ensuring the ternary mixture with the following proportion: 20% C. flexuosus, 53% C. carvi, and 27% A. calamus EOs. This antioxidant activity is higher than that exhibited by standard antioxidant BHT (IC50 = 191.13 ± 0.83 μg/mL).
Optimization of ABTS IC50
The contour plot and 3D surface (Fig. 4B) demonstrate that the suggested mixture for ABTS-IC50 response also contains the three studied EOs of C. flexuosus, C. carvi, and A. calamus. This combination yields ABTS-IC50 response value of approximately 82.25 μg/mL. Moreover, the desirability function in Fig. 5B supports this outcome by demonstrating that a mixture of 17% C. flexuosus, 43% C. carvi, and 40% A. calamus, leads to the best ABTS-IC50 value (60.48 μg/mL with 99.98% as the desirability percentage). These findings showed that the optimal combination of the three EOs exhibits strong scavenging ability against ABTS radical as compared to the standard BHT (IC50 = 114.07 ± 1.42 μg/mL).
The mixture design methodology has been adopted by several researchers in various disciplines, including the design of EOs mixtures. Baj et al.58 optimized the effect of a mixture containing Ocimum basilicum L., Cymbopogon nardus (L.) Rendle, Juniperus virginiana L., and Thymus vulgaris L. EOs to achieve high DPPH radical scavenging capacity. In this context, the combined effects of Petroselinum crispum (Mill.), Coriandrum sativum L., and Apium graveolens L. EOs were also optimized using the Simplex Lattice Mixture design57.
There has been an increasing interest in recent years to investigate the combined antimicrobial action of EOs by mixtures to achieve the highest antimicrobial effect27,59,60,61,62,63. Ouedrhiri et al.59 adopted the mixture design methodology to assess the possible synergistic potential of a combined treatment comprising Myrtus communis L., Thymus serpyllum L., and Artemisia herba-alba Asso EOs on B. subtilis, S. aureus and E. coli. Falleh et al.61 optimized the amounts of Lavandula stoechas L., Syzygium aromaticum (L.) Merr. & L.M. Perry, Myrtus communis L., and Cinnamomum zeylanicum Blume EOs by employing a mixture design technique. As a result, the optimal EOs mixture consisted of 59.4% C. zeylanicum, 38.2% L. stoechas and 2.4% S. aromaticum, which demonstrated synergistic interactions against E. coli.
Simultaneous optimization of all responses
In addition to its potential to provide accurate results for DPPHIC50 and ABTSIC50 responses individually, the desirability test also allows identification of the optimal conditions for both investigated responses simultaneously64. In our investigation, simultaneous optimization aims to determine the best compromise to enhance the DPPHHIC50 and ABTSIC50 responses. According to the desirability graph (Fig. 6), the optimization of the two studied responses was possible with a compromise percentage of 99.94% by ensuring the ternary combination with the following proportion: 18% C. flexuosus, 48% C. carvi, and 34% A. calamus EOs. The corresponding optimum response values for this formulation are ff187.79 μg/mL for DPPHIC50 and 64.55 μg/mL for ABTSIC50.
Experimental verification of the assumed model
The precision of the cubic models for the two responses, DPPHIC50 and ABTSIC50 was confirmed by a validation test. The trial involves the comparison of the expected outcomes with the experimental results. The selected test point data represent the EO proportions attained through simultaneous optimization of the two responses. As demonstrated in Table 6, the attained experimental values are strongly correlated with the estimated ones, and no significant difference was noticed between the estimated and experimental responses. The results confirm the accuracy of the choice of the assumed and verified models.
Conclusion
The use of natural formulations has garnered increasing interest. Essential oils possess a wide diversity of chemical constitutions depending on different factors, which may impact simultaneous interactions. Hence, to potentiate their mutual biological effect, statistical tools should be considered. Experimental mixture design may serve as an appropriate method to generate a combination with ideal activities useful for applications in the food or pharmaceutical sector. Herein, we deduce that the optimal antioxidant formulation consists of 20% C. flexuosus, 53% C. carvi, and 27% A. calamus for DPPHIC50. Whereas the combination of 17% C. flexuosus, 43% C. carvi, and 40% A. calamus is the best formulation leading to the highest scavenging activity against ABTS radical. This high activity may be related to their bioactive components. Nevertheless, further research on the pharmacokinetic and pharmacodynamics aspects as well as toxicological profile of these formulations is needed to ensure their safety and efficiency.
Plant collection approval
The authors confirm that no specific approval is needed to collect the studied plants in Morocco for research purposes.
IUCN policy statement T
The collection of plant material complies with relevant institutional, national, and international guidelines and legislation.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Phcog. Rev. 4, 118 (2010).
Stadtman, E. R. & Levine, R. L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25, 207–218 (2003).
Jaganjac, M., Tirosh, O., Cohen, G., Sasson, S. & Zarkovic, N. Reactive aldehydes—second messengers of free radicals in diabetes mellitus. Free Radic. Res. 47, 39–48 (2013).
Juan, C. A., Pérez Lastra, J. M., Plou, F. J. & Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22(9), 4642 (2021).
Pomatto, L. C. D. & Davies, K. J. A. Adaptive homeostasis and the free radical theory of ageing. Free Radical Biol. Med. 124, 420–430 (2018).
Phaniendra, A., Jestadi, D. B. & Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 30, 11–26 (2015).
Sharma, N. Free radicals, antioxidants and disease. Biol. Med. https://doi.org/10.4172/0974-8369.1000214 (2014).
Weber, R. W. Adverse Reactions to the Antioxidants Butylated Hydroxyanisole and Butylated Hydroxytoluene. In Food Allergy: Adverse Reactions to Foods and Food Additives (eds Metcalfe, D. D. et al.) 393–401 (Wiley, 2013).
Tortosa, V. et al. Computational methods for the identification of molecular targets of toxic food additives. Butylated hydroxytoluene as a case study. Molecules 25, 2229 (2020).
Al-Mijalli, S. H. et al. Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: A comprehensive In vitro and In silico study. Biochem. Syst. Ecol. 110, 104700 (2023).
Balahbib, A. et al. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 153, 112259 (2021).
Bouyahya, A. et al. Health benefits and pharmacological properties of carvone. Biomolecules 11, 1803 (2021).
Tit, D. M. & Bungau, S. G. Antioxidant activity of essential oils. Antioxidants 12(2), 383 (2023).
Hajlaoui, H. et al. Antimicrobial, antioxidant, anti-acetylcholinesterase, antidiabetic, and pharmacokinetic properties of Carum carvi L. and Coriandrum sativum L. essential oils alone and in combination. Molecules 26, 3625 (2021).
Loying, R. et al. Chemical compositions, in-vitro antioxidant, anti-microbial, anti-inflammatory and cytotoxic activities of essential oil of Acorus calamus L. rhizome from North–East India. J. Essent. Oil Bear. Plants 22, 1299–1312 (2019).
Abdelkader, H., Nadia, K. & Salima, B. Chemical composition and antioxidant potential of Pistacia lentiscus L. essential oil from Oran (Algeria). Adv. Biosci. Biotechnol. 7, 539 (2016).
Zahra, A. A., Hartati, R. & Fidrianny, I. Review of the chemical properties, pharmacological properties, and development studies of cymbopogon sp. Biointerface Res. Appl. Chem. 11, 10341–10350 (2020).
Avoseh, O., Oyedeji, O., Rungqu, P., Nkeh-Chungag, B. & Oyedeji, A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 20, 7438–7453 (2015).
Agrahari, P. & Singh, D. K. A review on the pharmacological aspects of Carum carvi. J. Biol. Earth Sci 4, 1–13 (2014).
Goyal, M., Gupta, V. K. & Singh, N. Carum carvi-an updated review. Indian J. Pharmac. Biol. Res. 6, 14–24 (2018).
Balakumbahan, R., Rajamani, K. & Kumanan, K. Acorus calamus: An overview. J. Med. Plants Res. 4, 2740–2745 (2010).
Rajput, S. B., Tonge, M. B. & Karuppayil, S. M. An overview on traditional uses and pharmacological profile of Acorus calamus Linn (Sweet flag) and other Acorus species. Phytomedicine 21, 268–276 (2014).
Khwairakpam, A. D. et al. Acorus calamus: A bio-reserve of medicinal values. J. Basic Clin. Physiol. Pharmacol. 29, 107–122 (2018).
Basavegowda, N. & Baek, K.-H. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules 11, 1267 (2021).
Sharma, K., Guleria, S., Razdan, V. K. & Babu, V. Synergistic antioxidant and antimicrobial activities of essential oils of some selected medicinal plants in combination and with synthetic compounds. Indus. Crops Prod. 154, 112569 (2020).
Purkait, S., Bhattacharya, A., Bag, A. & Chattopadhyay, R. R. Synergistic antibacterial, antifungal and antioxidant efficacy of cinnamon and clove essential oils in combination. Arch. Microbiol. 202, 1439–1448 (2020).
Benkhaira, N. et al. Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting https://doi.org/10.1016/j.bprint.2023.e00299 (2023).
Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry 5th edn. (Texensis Publishing, 2017).
Adams, R. P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy (Allured publishing corporation, 2001).
El Hachlafi, N. et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas, and Ammi visnaga. Biomed. Pharmacother. 164, 114937 (2023).
Hassan, W. N. F. W. et al. Mixture optimization of high-strength blended concrete using central composite design. Constr. Build. Mater. 243, 118251 (2020).
Tovar, L. P., Maciel, M. R., Pinto, G. M., Maciel Filho, R. & Gomes, D. R. Factorial design applied to concentrate bioactive component of Cymbopogon citratus essential oil using short path distillation. Chem. Eng. Res. Des. 88(2), 239–244 (2010).
Kursuncu, B. et al. Optimization of foam concrete characteristics using response surface methodology and artificial neural networks. Constr. Build. Mater. 337, 127575 (2022).
Soussi, M., Fadil, M., Yaagoubi, W. A., Benjelloun, M. & El Ghadraoui, L. Simultaneous optimization of phenolic compounds and antioxidant abilities of moroccan Pimpinella anisum extracts using mixture design methodology. Processes 10, 2580 (2022).
Candioti, L. V., De Zan, M. M., Cámara, M. S. & Goicoechea, H. C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 124, 123–138 (2014).
Szczeblewski, P. et al. The role of centrifugal partition chromatography in the removal of β-asarone from Acorus calamus essential oil. Sci. Rep. 12, 22217 (2022).
Uebel, T., Hermes, L., Haupenthal, S., Müller, L. & Esselen, M. α-Asarone, β-asarone, and γ-asarone: Current status of toxicological evaluation. J. Appl. Toxicol. 41, 1166–1179 (2021).
Ghannay, S., Aouadi, K., Kadri, A. & Snoussi, M. GC-MS profiling, vibriocidal, antioxidant, antibiofilm, and anti-quorum sensing properties of Carum carvi L. essential oil: In vitro and in silico approaches. Plants 11, 1072 (2022).
Lasram, S. et al. Antifungal and antiaflatoxinogenic activities of Carum carvi L., Coriandrum sativum L. seed essential oils and their major terpene component against Aspergillus flavus. Indus Crops Prod. 134, 11–18 (2019).
Gao, S. et al. Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell. Infect. Microbiol. 10, 603858 (2020).
Pathania, D. et al. Exploring phytochemical composition, photocatalytic, antibacterial, and antifungal efficacies of Au NPs supported by Cymbopogon flexuosus essential oil. Sci. Rep. 12, 14249 (2022).
El Hachlafi, N. et al. Exploration of novel antibacterial and anti-adhesive formulations from three chemically characterized essential oils: Optimization using experimental design methodology. Sci. Afr. 22, e01927 (2023).
Liu, R. & Mabury, S. A. Synthetic phenolic antioxidants: A review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 54, 11706–11719 (2020).
Cecerska-Heryć, E. et al. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 93, 1–8 (2021).
Aly, A., Maraei, R., Rezk, A. & Diab, A. Phytochemical constitutes and biological activities of essential oil extracted from irradiated caraway seeds (Carum carvi L.). Int. J. Radiat. Biol. 99, 318–328 (2023).
Khanal, S., Bhandari, D. P., Bhandari, L., Dangol, S. & Adhikari, A. Chemical profiling and antioxidant activities of essential oil from the rhizomes of Acorus calamus L.. Bibechana 17, 89–95 (2020).
Caballero-Gallardo, K., Quintero-Rincón, P., Stashenko, E. E. & Olivero-Verbel, J. Photoprotective agents obtained from aromatic plants grown in Colombia: Total phenolic content, antioxidant activity, and assessment of cytotoxic potential in cancer cell lines of Cymbopogon flexuosus L. and Tagetes lucida Cav essential oils. Plants 11, 1693 (2022).
Rezende, D. et al. Bactericidal and antioxidant effects of essential oils from Satureja montana L., Myristica fragrans H. and Cymbopogon flexuosus. Lett. Appl. Microbiol. 74(5), 741–751 (2022).
Amorati, R., Foti, M. C. & Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 61, 10835–10847 (2013).
Dawidowicz, A. L. & Olszowy, M. Does antioxidant properties of the main component of essential oil reflect its antioxidant properties? The comparison of antioxidant properties of essential oils and their main components. Nat. Prod. Res. 28, 1952–1963 (2014).
Diniz Nascimento, L. et al. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 10, 988 (2020).
Pina, L. T. et al. Carvone and its pharmacological activities: A systematic review. Phytochemistry 196, 113080 (2022).
Ling, Q. et al. Chemical composition and antioxidant activity of the essential oils of citral-rich chemotype Cinnamomum Camphora and Cinnamomum Bodinieri. Molecules 27, 7356 (2022).
Shi, C. et al. Antimicrobial, antioxidant, and antitumor activity of epsilon-poly-L-lysine and citral, alone or in combination. Food Nutr. Res. 60, 31891 (2016).
Voinovich, D., Campisi, B., Phan-Tan-Luu, R. & Beal, A. Experimental design for mixture studies. In Comprehensive Chemometrics, 2nd edn (eds Brown, S. et al.) 327–383 (Elsevier, 2020)
Aboulghazi, A., Bakour, M., Fadil, M. & Lyoussi, B. Simultaneous optimization of extraction yield, phenolic compounds and antioxidant activity of Moroccan propolis extracts: Improvement of ultrasound-assisted technique using response surface methodology. Processes 10, 297 (2022).
Nouioura, G. et al. Optimization of a New antioxidant formulation using a simplex lattice mixture design of Apium graveolens L., Coriandrum sativum L., and Petroselinum crispum M. Grown in Northern Morocco. Plants 12, 1175 (2023).
Baj, T. et al. Synergistic Antioxidant activity of four—component mixture of essential oils: Basil, cedarwood, citronella and thyme for the use as medicinal and food ingredient. Antioxidants 12, 577 (2023).
Ouedrhiri, W. et al. Optimized antibacterial effects in a designed mixture of essential oils of Myrtus Communis, Artemisia Herba-alba and Thymus Serpyllum for wide range of applications. Foods 11, 132 (2022).
Ouedrhiri, W. et al. Mixture design of Origanum compactum, Origanum majorana and Thymus Serpyllum essential oils: Optimization of their antibacterial effect. Indus. Crops Prod. 89, 1–9 (2016).
Falleh, H. et al. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J. Food Process. Preserv. 43, e14257 (2019).
Souiy, Z. et al. Application of simplex-centroid design methodologies to optimize the anti-bacterial and anti-candidal activity of the mixture of Menthapulegium, Pituranthos Chloranthus and Thymus Algeriensis essential oils. Med. Aromat. Plants 10, 365 (2021).
Kachkoul, R. et al. The synergistic effect of three essential oils against bacteria responsible for the development of Lithiasis infection: An optimization by the mixture design. Evid. Complement. Altern. Med. 2021, 1 (2021).
Marinkovic, V. A novel desirability function for multi-response optimization and its application in chemical engineering. Chem. Indus. Chem. Eng. Q 26, 309–319 (2020).
Acknowledgements
The authors extend their appreciation to the Deanship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number: IFP22UQU4310026DSR074.
Author information
Authors and Affiliations
Contributions
A. B. and B. H. G. conceptualized the study and design of the experimental process. H. A. and M. J. conducted the antioxidant assays. H. N. M. and A. E. performed chemical extraction and characterization. A. Q. and A. A. worked on the simplex centroid design and performed the statistical analysis. K. W. G. refined statistical and computational models. S. L. T. performed data curation. N. E. H. supervised the overall study. All authors contributed equally in the manuscript writing, review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assaggaf, H., Jeddi, M., Mrabti, H.N. et al. Design of three-component essential oil extract mixture from Cymbopogon flexuosus, Carum carvi, and Acorus calamus with enhanced antioxidant activity. Sci Rep 14, 9195 (2024). https://doi.org/10.1038/s41598-024-59708-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59708-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.