Abstract
The invasive Asian longhorned tick Haemaphysalis longicornis that vectors and transmits several animal pathogens is significantly expanding in the United States. Recent studies report that these ticks also harbor human pathogens including Borrelia burgdorferi sensu lato, Babesia microti, and Anaplasma phagocytophilum. Therefore, studies that address the interactions of these ticks with human pathogens are important. In this study, we report the characterization of H. longicornis organic anion-transporting polypeptides (OATPs) in interactions of these ticks with A. phagocytophilum. Using OATP-signature sequence, we identified six OATPs in the H. longicornis genome. Bioinformatic analysis revealed that H. longicornis OATPs are closer to other tick orthologs rather than to mammalian counterparts. Quantitative real-time PCR analysis revealed that OATPs are highly expressed in immature stages when compared to mature stages of these ticks. In addition, we noted that the presence of A. phagocytophilum upregulates a specific OATP in these ticks. We also noted that exogenous treatment of H. longicornis with xanthurenic acid, a tryptophan metabolite, influenced OATP expression in these ticks. Immunoblotting analysis revealed that antibody generated against Ixodes scapularis OATP cross-reacted with H. longicornis OATP. Furthermore, treatment of H. longicornis with OATP antibody impaired colonization of A. phagocytophilum in these ticks. These results not only provide evidence that the OATP-tryptophan pathway is important for A. phagocytophilum survival in H. longicornis ticks but also indicate OATP as a promising candidate for the development of a universal anti-tick vaccine to target this bacterium and perhaps other rickettsial pathogens of medical importance.
Similar content being viewed by others
Introduction
Haemaphysalis longicornis, also known as the Asian longhorned tick, is a hard tick and native in eastern Asia, Australia, New Zealand, and several pacific islands1,2. The first recognized human bite of H. longicornis was reported in 20193. This tick is reported to vector at least 30 human pathogens4. In Asia, H. longicornis ticks have been reported to serve as vector for various pathogens like spotted fever group rickettsia, Anaplasma species, Borrelia burgdorferi sensu lato, and Babesia species5,6. This tick was recently found in the United States7. The detection of human pathogenic variant of A. phagocytophilum was first reported in H. longicornis that were field collected in Pennsylvania, USA8. A recent study reported that 8% of field collected H. longicornis ticks from Ohio, USA, were positive for A. phagocytophilum9. In addition, another recent study reported the presence of B. burgdorferi sensu lato and B. microti in field collected ticks from Pennsylvania, USA10. More recent reports show that Ehrlichia chaffeensis, Anaplasma bovis and spotted fever group rickettsiae were also detected in H. longicornis ticks11,12. Recently, human pathogenic A. phagocytophilum variant was detected in field collected H. longicornis ticks in USA8,9. Haemaphysalis longicornis ticks have four stages of development (egg, larvae, nymph, adult). Larvae, nymphs and adults require a blood meal to molt. These ticks could feed on a wide variety of hosts including rodents, livestock, carnivores, and birds1. Haemaphysalis longicornis ticks can reproduce bisexually or asexually by parthenogenesis1,2,13 making them highly invasive species. Modeling study has predicted that eastern North America from southern Canada to the Gulf Coast, small temperate area on the West Coast, Midwestern and Southern United States habitats are suitable for H. longicornis14. Due to the ability of this tick to transmit multiple pathogens to wide range of vertebrate hosts including pets and livestock, the invasion and dominance of H. longicornis infestations are of medical and veterinary concern.
Anaplasma phagocytophilum, an obligate intracellular bacterium, is a causative agent of human granulocytic anaplasmosis (HGA)15,16. In the United States, I. scapularis, also known as a deer tick, and Ixodes pacificus are known vectors to transmit A. phagocytophilum to the vertebrate hosts17,18,19,20. This bacterium infects diverse hosts and is reported to modulate various signaling cascades in ticks and in vertebrates hosts17,21,22,23,24,25,26,27,28. Organic anion transporting polypeptides (OATPs) are transmembrane proteins that aid in the movement of various anions, hormones, drugs, signaling molecules, growth factors and toxins29,30,31,32,33. Our previous studies reported that A. phagocytophilum modulates I. scapularis OATPs and tryptophan pathway not only for its survival but also for its transmission from these ticks to vertebrate host34,35,36,37,38,39,40. Anaplasma phagocytophilum upregulates endogenous production of xanthurenic acid (XA), a tryptophan metabolite37, increases kynurenine amino transferase (KAT) activity34,40, upregulates expression of IsOATP4056 transcripts and protein36,40, modulates ROS production34 and activates p38 MAPK pathway for its survival in the vector host37. We have previously noted that exogenously added XA can induce isoatp4056 expression and increases the bacterial burden in A. phagocytophilum infected I. scapularis ticks and tick cells40. In addition, we reported that OATPs are important for survival of tick-borne viruses in I. scapularis ticks41. Overall, these studies indicate that OATPs are important for intracellular pathogen survival in I. scapularis ticks.
OATPs have several transmembrane domains, intracellular and extracellular loops36,41. In our previous study, we generated affinity purified antibodies against the C-terminal extra-cellular loop 6 (EL-6) of I. scapularis IsOATP4056 protein36. We reported that when mice were passively immunized with an anti-EL6 antibody there was a significant decrease in the transmission of A. phagocytophilum from infected ticks to the murine host36. In addition, we reported that ticks ingested EL-6 antibody via a blood meal. Anti-EL6 antibodies in a blood meal affected A. phagocytophilum loads in fed ticks36. We also noted that treatment of tick cells with an anti-EL6 antibody affected A. phagocytophilum growth in these cells36. Collectively, these results not only indicate that anti-EL6 antibodies could be effective in impairing transmission of A. phagocytophilum from infected ticks to the naïve vertebrate host but also aid in clearing bacterial loads in ticks.
In this study, we not only provide evidence that H. longicornis ticks expresses OATPs, but also report that these OATPs are modulated by A. phagocytophilum in these ticks. We also report a method for the generation of A. phagocytophilum-infected H. longicornis ticks in vitro. We noted that treatment of H. longicornis unfed nymphs with an anti-EL6 antibody decreased bacterial burden in these ticks. This study provide evidence that EL6 region of OATP could be envisioned as an important candidate for the development of universal anti-vector vaccine to target A. phagocytophilum and perhaps other intracellular pathogens in hard ticks.
Results
Expression of H. longicornis organic anion transporting polypeptides (OATPs) transcripts
OATPs have a signature motif (WxGxWWxG) in the amino acid sequence41. We first screened the genome of H. longicornis and identified six OATPs (Supplementary Table 1). To analyze whether H. longicornis ticks express these OATPs, RNA from unfed uninfected adult ticks was used as a template for reverse transcription polymerase chain reaction (RT-PCR). Using oligonucleotides mentioned in Supplementary Table 2, we amplified all six OATP transcripts (Fig. 1A). The RT-PCR products were excised and sequenced to confirm the nucleotide sequence of all six OATPs. Amino acid percent identity analysis of H. longicornis OATP sequence with other ortholog proteins from Dermacentor andersoni (Da), Hyalomma asiaticum (Ha), Homo sapiens (Hs), I. scapularis (Is), Mus musculus (Mm) and Rhipicephalus sanguineus (Rs) was determined by CLUSTALW alignment (Fig. 1B). The GenBank accession numbers for these proteins are shown in Supplementary Table 3. The H. longicornis OATPs (KAH9381028.1, KAH9381027.1, KAH9381876.1, KAH9365504.1, KAH9380884.1, KAH9381025.1) share 62–88% identity with OATPs from other ticks analyzed in this study but 24–42% identity with OATPs from human and mice (Fig. 1B, Supplementary Figs. 1 and 2). Furthermore, phylogenetic analyses revealed that H. longicornis OATP amino acid sequences fall in the same clade or clade close to OATPs from other ticks (Supplementary Figs. 3 and 4). Both human and mouse OATPs form a different clade (Supplementary Figs. 3 and 4). These results reveal that H. longicornis OATPs are similar to OATPs from other ticks.
PCR amplification and sequence analysis of H. longicornis oatp genes. (A) PCR amplification products of H. longicornis oatps is shown. M indicates the DNA marker and NTC indicates no template control. T indicates cDNA template generated from unfed H. longicornis nymphal tick RNA. GenBank accession numbers are labeled at the top of the gel image. (B) Bar graphs (with different color shades) represents percent identity of H. longicornis OATPs amino acid sequence with ortholog proteins from Dermacentor andersoni (Da), Hyalomma asiaticum (Ha), Homo sapiens (Hs), I. scapularis (Is), Mus musculus (Mm) and Rhipicephalus sanguineus (Rs). GenBank accession numbers for all OATPs are shown in Supplementary Table 3.
Comparison of OATP-signature sequence and prediction of posttranslational modifications on H. longicornis OATPs
The alignment of the amino acid sequences of H. longicornis OATPs with the OATP signature sequence WxGxWWxG41 using ClustalW program revealed a conserved OATP signature motif in all six H. longicornis OATPs (Fig. 2A). Bioinformatic analysis of post translational modification sites in H. longicornis OATPs revealed that KAH9381027.1 had higher number of N-myristoylation sites (20 sites) and cAMP- and cGMP-dependent phospho sites (five sites) compared to other OATPs (Fig. 2B). KAH9381876.1 OATP had higher number of casein kinase II phospho sites (15 sites) and N-glycosylation (eight sites). KAH9381025.1 had the higher number of protein kinase C phospho sites (14 sites) compared to other OATPs. All but XP_040071371 of I. scapularis OATP orthologs had at least one of each of these posttranslational modification sites (Supplementary Table 4). Collectively, these analyses show that like I. scapularis, H. longicornis OATPs also have several posttranslational modification sites in their amino acid sequences.
Alignment of H. longicornis OATPs with the OATP-signature sequence and analysis of post-translational modifications on H. longicornis OATPs. (A) CLUSTALW alignment of consensus OATP-signature sequence (WxGxWWxG) with all H. longicornis OATPs. Alignment was performed with DNASTAR MEGALIGN software. Residues that match are shaded in black. (B) Number of predicted post-translational sites (shown on X-axis) in H. longicornis OATPs were analyzed at PROSITE. Histograms represent the number of N- myristoylation, casein kinase II phosphorylation, N-glycosylation, cAMP or c-GMP-dependent protein kinase phosphorylation and protein kinase C phosphorylation sites for each OATP. Full-length H. longicornis OATP sequences were considered to determine posttranslational modification sites.
Expression of H. longicornis OATPs is developmentally regulated
We then analyzed whether all six OATP transcripts are expressed in different tick developmental stages (Fig. 3). qRT-PCR analysis showed that larvae expressed significantly (P < 0.05) higher levels of kah9381028.1 (Fig. 3A), kah9381027.1 (Fig. 3B), kah9381876.1 (Fig. 3C), kah9365504.1 (Fig. 3D), kah9380884.1 (Fig. 3E), kah9381025.1 (Fig. 3F) in comparison to levels noted in adult ticks. Larvae expressed significantly (P < 0.05) higher levels of kah9381028.1, kah9381876.1 and kah9380884.1 mRNA in comparison to nymphs (Fig. 3A, C, E). No significant (P > 0.05) differences in H. longicorinis oatp mRNA levels were observed between nymph and adults (Fig. 3A–F). However, we observed a decreased trend in the expression of OATPs in adults compared to nymphs (Fig. 3A–F). These data show variable expression pattern of OATP transcript levels at different developmental stages of H. longicornis ticks.
Expression of H. longicornis oatp transcripts at different tick developmental stages. Quantitative RT-PCR analysis showing expression of kah9381028.1 (A), kah9381027.1 (B), kah9381876.1 (C), kah9365504.1 (D), kah9380884.1 (E), kah9381025.1 (F) at different tick developmental stages in uninfected unfed H. longicornis. Each data point in nymphs and adult samples represents transcript levels noted in an individual tick. Closed circles, squares and triangles represent larvae, nymphs and adults, respectively. For larval samples, each data point represents transcript levels noted in five pooled larvae. Horizontal lines in the graphs represent the mean value of the data points. The mRNA levels of these genes were normalized to tick beta-actin mRNA levels. Statistical significance was calculated using ANOVA analysis.
Generation of A. phagocytophilum-infected H. longicornis ticks in vitro
Pathogens like Ehrlichia chaffeensis, A. bovis11, Lyme spirochetes and spotted fever group rickettsiae12 have been detected in H. longicornis ticks. Recently, human pathogenic A. phagocytophilum variant was detected in field collected H. longicornis ticks in USA8,9. We reasoned whether expression of OATPs in H. longicornis is altered upon A. phagocytophilum infection. Anaplasma phagocytophilum-infected H. longicornis ticks were generated in vitro as shown in the schematic diagram (Fig. 4A). Briefly, dense core (DC) form of A. phagocytophilum was isolated from infected HL-60 cultures as described42. Uninfected unfed H. longicornis nymphal ticks were bathed in A. phagocytophilum DC culture for 40 min (Fig. 4A). Ticks were washed and incubated for 3, 5, 7 and 10 days and processed for DNA extraction. qPCR was performed with the DNA isolated from these bathed and washed ticks to detect A. phagocytophilum loads. The qPCR analysis revealed no significant (P > 0.05) differences in the bacterial loads between day 3 and day 5 post-infected ticks (Fig. 4B). However, significantly (P < 0.05) higher bacterial loads were evident in both day 7 and day 10 post-infected ticks in comparison to day 3 and day 5 post-infected ticks (Fig. 4B). No significant (P > 0.05) differences in the bacterial loads were evident between day 7 and day 10 post-infected (p.i.) ticks (Fig. 4B). The results not only indicate day 7 as a peak of infection time point in the in vitro A. phagocytophilum infection of H. longicornis ticks but also suggests that A. phagocytophilum multiply in H. longicornis ticks.
Anaplasma phagocytophilum up-regulates expression of kah9381876.1 mRNA levels in unfed H. longicornis nymphs. (A) Schematic representation showing in vitro infection of H. longicornis nymphal ticks with A. phagocytophilum. (B) qPCR with tick DNA showing levels of A. phagocytophilum in infected H. longicornis ticks at different days post-infection. Statistical significance was calculated using ANOVA analysis. qRT-PCR analysis showing expression of kah9381028.1 (C), kah9381027.1 (D), kah9381876.1 (E), kah9365504.1 (F), kah9380884.1 (G), kah9381025.1 (H) in unfed uninfected or A. phagocytophilum-infected H. longicornis ticks. Open circles represent uninfected (UI) and closed circles represent infected (I) ticks. Each circle represents data from samples generated from one tick. Horizontal lines in the graphs represent the mean value of the data points. The mRNA levels of these genes were normalized to tick beta-actin mRNA levels. P value from student’s t-test is shown.
We then used I. scapularis ticks which is a known vector for this pathogen as a control group and performed in vitro infection like the one performed with H. longicornis ticks. The in vitro infected I. scapularis was incubated for 7 days post infection. qPCR analysis followed by agarose gel electrophoresis confirmed that like H. longicornis, I. scapularis ticks could also be infected with A. phagocytophilum by bathing these ticks in isolated DC cultures (Supplementary Fig. 5A, B). However, qPCR analysis revealed no significant difference in the A. phagocytophilum loads between I. scapularis and H. longicornis ticks at day 7 p.i. (Supplementary Fig. 5B).
Anaplasma phagocytophilum induces expression of specific H. longicornis OATPs in unfed ticks
The impact of tick-borne pathogens on the expression of H. longicornis OATPs is not studied. Therefore, expression of OATPs was determined in the RNA samples generated from unfed H. longicornis nymphal ticks infected with A. phagocytophilum and compared the levels in uninfected ticks (Fig. 4C–H). qRT-PCR analysis revealed no significant differences in the expression levels of kah9381028.1 (Fig. 4C), kah9381027.1 (Fig. 4D), kah9365504.1 (Fig. 4F), kah9380884.1 (Fig. 4G) and kah9381025.1 (Fig. 4H) between unfed uninfected ticks and A. phagocytophilum-infected ticks. However, expression of kah9381876.1 (Fig. 4E) was significantly (P < 0.05) upregulated in the presence of A. phagocytophilum in comparison to the uninfected controls. These results show that A. phagocytophilum induces expression of specific tick OATPs (kah9381876.1) in unfed H. longicornis ticks.
Xanthurenic acid (XA), a tryptophan metabolite, induces some of the OATPs expression in A. phagocytophilum-infected H. longicornis unfed nymphal ticks
In our previous study, we noted higher levels of XA in A. phagocytophilum-infected I. scapularis ticks37. Therefore, we studied whether XA has any impact on bacterial burden and OATP expression in A. phagocytophilum-infected H. longicornis ticks. First, we generated A. phagocytophilum-infected H. longicornis ticks (day 7 post-infected) as described in Fig. 4A. These A. phagocytophilum-infected H. longicornis nymphs were bathed in 100 µM XA or mock solutions as depicted in the schematic diagram (Fig. 5A). After 24 h, post-bathed ticks were washed and processed for DNA or RNA extractions. qPCR with DNA samples revealed significantly (P < 0.05) increased bacterial burden in XA-treated A. phagocytophilum-infected H. longicornis ticks compared to the levels noted in mock-treated control ticks (Fig. 5B). qRT-PCR analysis with RNA samples revealed no significant differences in the expression levels of kah9381027.1 (Fig. 5D) between mock- and XA-treated A. phagocytophilum-infected ticks. However, expression of kah9381876.1 (Fig. 5E), kah9365504.1 (Fig. 5F) and kah9380884.1 (Fig. 5G) were significantly (P < 0.05) upregulated in XA-treated A. phagocytophilum-infected H. longicornis ticks in comparison to the mock-treated controls. Conversely, expression of kah9381028.1 (Fig. 5C) and kah9381025.1 (Fig. 5H) were significantly (P < 0.05) down-regulated in XA-treated A. phagocytophilum-infected H. longicornis ticks in comparison to the respective mock-treated controls. These results show that XA modulates expression of OATPs in A. phagocytophilum-infected H. longicornis ticks.
Exogenous treatment with XA differentially regulates some of the oatps in A. phagocytophilum-infected unfed H. longicornis nymphs. (A) Schematic representation showing the procedure for exogenous XA treatment (100 μM) of A. phagocytophilum infected H. longicornis nymphs. (B) qPCR with tick DNA showing bacterial burden in mock or XA-treated A. phagocytophilum-infected H. longicornis ticks. qRT-PCR analysis showing expression of kah9381028.1 (C), kah9381027.1 (D), kah9381876.1 (E), kah9365504.1 (F), kah9380884.1 (G), kah9381025.1 (H) in unfed A. phagocytophilum-infected H. longicornis ticks upon treatment with XA. Mock controls were treated with the same amount of solvent used for the preparation of XA. Open circles represent mock (M) and closed circles represent xanthurenic acid (XA)-treated A. phagocytophilum infected ticks. Each circle represents data from samples generated from one tick. Horizontal lines in the graphs represent the mean value of the data points. The mRNA levels of these genes were normalized to tick beta-actin mRNA levels. P value from student’s t-test is shown.
Treatment with EL-6 antibody affects A. phagocytophilum burden in H. longicornis ticks
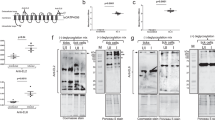
Affinity purified polyclonal antibody was generated against IsOATP4056 Extracellular Loop-6 (EL-6) region36 (Fig. 6A). We reported that passive immunization with EL-6 antibody impaired A. phagocytophilum transmission from infected ticks to the naïve murine hosts36. In addition, treatment with EL-6 antibody affected A. phagocytophilum burden in tick cells36. H. longicornis OATP (kah9381876.1) is an ortholog of IsOATP4056. In this study, we noted that kah9381876.1, was significantly upregulated upon A. phagocytophilum infection in H. longicornis ticks (Fig. 4E). The epitope for EL-6 antibody binding in I. scapularis IsOATP405636 is highly conserved in H. longicornis KAH9381876.1. Therefore, we used an anti-EL-6 antibody and performed immunoblotting and antibody-blocking experiments. Immunoblotting analyses with EL-6 antibody and 12% (Fig. 6B) or 8% (Supplementary Fig. 6) SDS-PAGE performed in non-reducing (no boiling) and reducing conditions revealed an intense band above 250 kDa in H. longicornis (Hl) whole tick lysates (Fig. 6B). As expected, and based on our previous observation36, we noted intense band above 250 kDa in I. scapularis (Is) whole tick lysate (Fig. 6B and Supplementary Fig. 6). These results show that anti-EL-6 antibody also recognizes H. longicornis OATP protein (KAH9381876.1). We then explored whether treatment with anti-EL6 antibody has any effect on A. phagocytophilum burden in H. longicornis ticks. We noticed significant reduction in the bacterial burden in A. phagocytophilum-infected H. longicornis ticks treated with EL-6 antibody when compared to control IgG-treated groups (Fig. 6C). In summary, these results indicate that treatment with anti-EL-6 antibody impairs A. phagocytophilum colonization in H. longicornis ticks.
EL-6 antibody-treatment reduces bacterial burden in A. phagocytophilum-infected unfed H. longicornis nymphs. (A) Schematic representation of I. scapularis OATP organization on the tick cell plasma membrane is shown. Extracellular loops are indicated with numbers from 1 to 6. The N- and C-terminal ends are labeled. Antibody generated against epitope from I. scapularis EL-6 region was used in this study. Schematics are not drawn to the scale. (B) Immunoblotting analysis with EL-6 antibody showing levels of KAH9381876.1 or IsOATP4056 (indicated by black arrow) in uninfected unfed H. longicornis and I. scapularis tick lysates, respectively is shown. M indicates protein marker. Non red: indicates non-reducing conditions. Red: indicates reducing conditions, Hl indicates H. longicornis, and Is indicates I. scapularis. Ponceau S-stained gel image for total protein profile serves as loading control in the immunoblotting analysis. (C) qRT-PCR analysis showing bacterial burden (analyzed for A. phagocytophilum p44 gene DNA levels) in control IgG or EL-6 antibody-treated A. phagocytophilum-infected unfed H. longicornis ticks. Control IgG and EL-6 antibodies were used at a concentration of 5 µg/ml. Open circles represent samples generated from control IgG-treated and closed circles represent EL-6 -treated A. phagocytophilum-infected unfed H. longicornis ticks. Each circle represents data from samples generated from one tick. Horizontal lines in the graphs represent the mean value of the data points. A. phagocytophilum p44 levels were normalized to tick actin levels. P value from student’s t-test is shown.
Discussion
Organic anion transporting polypeptides (OATPs) are a family of highly conserved transporters in arthropods like ticks, lice, and mosquitoes41. Ixodes scapularis tick express nine OATPs41,43. Our previous studies indicated that OATPs are critical for A. phagocytophilum survival and transmission from these ticks to the vertebrate host34,36,37,39,40. Ixodes scapularis and I. pacificus are the common vectors for A. phagocytophilum17,18,19,20. However, recent studies have indicated the presence of A. phagocytophilum in H. longicornis ticks collected from the field8,9. In this study, we noted that H. longicornis OATPs are also important for A. phagocytophilum survival in these ticks.
The role of H. longicornis molecules in the interactions with A. phagocytophilum is not known. As OATPs are highly conserved in different ticks and are critical for A. phagocytophilum survival in I. scapularis ticks, we reasoned whether these important molecules have any role in the interactions of H. longicornis ticks with this bacterium. Screening of H. longicornis genome with OATP signature sequence (WXGXWWXG) resulted in the identification of six OATPs. Along with the conserved tryptophan and glycine residues, we noted that all six H. longicornis OATPs have conserved alanine residues in the OATP signature sequence. The significance of this conserved alanine residue in the OATP signature sequence is currently not understood. In addition to the six OATPs analyzed in this study, we also noted that a hypothetical protein (GenBank Acc. No. KAH9381873.1) was annotated as OATP. However, OATP signature sequence was absent in this sequence. Therefore, we excluded KAH9381873.1 from the current analysis. High degree of percent identity of H. longicornis OATPs with other tick OATPs, including I. scapularis, suggests a conserved functional role for these important molecules in various ticks.
We noted variable levels of OATP transcripts at different developmental stages of H. longicornis ticks. The observation of significantly lower trend of all analyzed H. longicornis OATP transcripts in adult ticks compared to the levels noted in larvae or nymphal stages of these ticks suggest that these molecules are important in the immature stages of ticks. Larval and nymphal H. longicornis ticks acquire pathogens by feeding on infected animals. In addition, OATPs are important for blood feeding36,44. Therefore, it could be reasoned that increased levels of H. longicornis OATP expression in larval or nymphal ticks could facilitate blood feeding and/or acquisition and transmission of pathogens from and to the vertebrate host, respectively.
Haemaphysalis longicornis OATPs contain several posttranslational sites in their amino acid sequences including glycosylation sites. In our previous study, we noted that I. scapularis OATP (IsOATP4056) that has a predicted molecular mass of ~ 100 kDa was detected at above 250 kDa in an immunoblot performed with EL-6 and EL-2 antibody36. Treatment with deglycosylation mix did not impact the molecular mass of IsOATP4056 suggesting that this protein could exist in an oligomerized form or in other posttranslational-modified form36. Haemaphysalis longicornis OATP (GenBank Acc. No. KAH9381876) is an ortholog of IsOATP4056. The epitope region that was selected for the generation of EL-6 antibody is highly conserved in H. longicornis OATP (GenBank Acc. No. KAH9381876). Therefore, the observation of H. longicornis OATP (GenBank Acc. No. KAH9381876) at more than 250 kDa in an immunoblot suggests that this OATP may also be present in oligomerized and/or posttranslational-modified form. The observation of band above 250 kDa when SDS-PAGE analysis was performed with both 8% or 12% gel and in non-reducing and no-boiling conditions further supports that H. longicornis OATP (GenBank Acc. No. KAH9381876) may exist as an oligomerized form. In addition, the observation of multiple bands in both H. longicornis and I. scapularis total protein lysates (performed in both non-reducing and reducing conditions) is consistent with the observation of mammalian OATPs that forms dimers/homo or hetero-oligomers45,46. Furthermore, the presence of N-myristoylation sites, casein kinase II phospho sites, N-glycosylation sites, cAMP-and cGMP-dependent phospho sites or protein kinase C phospho sites indicate multiple kinds of posttranslational modifications alone or in combination could also contribute for the increased molecular mass of H. longicornis OATP (GenBank Acc. No. KAH9381876).
Our previous studies reported that there is a strong interplay between OATPs and tryptophan pathway34,35,36,37,39,40,41. We noted that exogenous addition of XA upregulated isoatp4056 expression40. Knockdown of kynurenine amino transferase (KAT, enzyme that catalyzes formation of XA), via RNAi impacted isoatp4056 expression40. We also reported that XA indirectly could facilitate transcriptional activation of isoatp4056 gene at the promoter level40. These data prompted us to explore whether XA has any impact on H. longicornis OATP’s expression. Our results indicated differential regulation of H. longicornis OATPs. Interestingly, the expression of H. longicornis OATP (GenBank Acc. No. KAH9381876.1) was upregulated upon exogenous treatment with XA. These results indicate a conserved role for XA and OATP pathway in tick-A. phagocytophilum interactions. Some H. longicornis OATPs (GenBank Acc. Nos. KAH9381028.1 and KAH9381025.1) were noted to be downregulated in the presence of exogenous XA. However, H. longicornis OATPs (GenBank Acc. Nos. KAH9381028.1 and KAH9381025.1) transcript levels were not altered upon A. phagocytophilum infection when compared to the levels noted in the uninfected controls. The significance of the downregulation of these genes in the presence of exogenous XA is not known. Future studies on these aspects would provide more details on the role of XA in different physiological processes in these ticks.
We noted significantly decreased A. phagocytophilum loads in I. scapularis ticks fed on mice passively immunized with EL-6 antibodies36. In addition, we noted significant decrease in A. phagocytophilum loads in ISE6 tick cells upon treatment with EL-6 antibody36. The observation of reduced A. phagocytophilum burden in H. longicornis ticks upon treatment with EL-6 antibody further supports our previous observations with I. scapularis ticks and ISE6 tick cell line36. Moreover, these data support that IsOATP4056 and KAH9381876.1 that are orthologs in I. scapularis and H. longicornis ticks, respectively, aids in the survival/colonization of A. phagocytophilum in both these ticks. Our future studies are aimed in deciphering significance of OATP-tryptophan pathways in H. longicornis-pathogen interactions.
Haemaphysalis longicornis ticks are dramatically expanding their colonies in the United States. Studies like these are not only important to address some of the important vector biological questions but also could lead us in the development of strategies to target H. longicornis ticks and pathogens they transmit. In summary, our study suggests that treatment with EL-6 antibody could be an ideal strategy to target A. phagocytophilum colonization in different ticks.
Methods
Bacteria
Anaplasma phagocytophilum isolate NCH-1 (obtained from BEI Resources, NIAID, NIH), referred as A. phagocytophilum, was used throughout this study. Anaplasma phagocytophilum was maintained in HL-60 cells and isolated from these cells as described37,42. Briefly, infected HL-60 cells were centrifuged for 10 min at 2300 × g at 4 °C and the pellet was washed twice with sterile 1× phosphate buffer saline. Cells were then re-suspended in 4 ml 1× PBS and subjected to 8 quick pulses of sonication (8 s burst interspersed with 8 s rest periods using an ultrasonic sonicator on an amplitude setting of 30). Cells were then passed through 27-gauge needles for 6–8 times followed by freezing at − 80 °C for 15 min. After thawing, cells were centrifuged for 5 min at 700 × g at 4 °C and the supernatant was transferred to a fresh tube. The supernatant was centrifuged for 5 min at 1000 × g at 4 °C and the resulting supernatant was transferred to another fresh tube. This supernatant was then centrifuged for 10 min at 2300 × g at 4 °C. The pellet of host-cell free A. phagocytophilum (dense core, DC) was resuspended in 100 μl of 1× PBS and used for in vitro infection.
Ticks
Haemaphysalis longicornis ticks (larvae, nymphs, adults) used in this study were obtained from BEI resources, CDC. Tick rearing was conducted in an Environmental Chamber from Parameter Generation and Control, USA. The incubator was set at 23 ± 2 °C with 94% relative humidity and 14:10 light:dark conditions. Ixodes scapularis (larvae) were obtained from BEI resources, CDC, and fed on uninfected C57BL/6J mice. Fed larvae were allowed to molt into nymphs. Total lysates from unfed nymphs were used in the immunoblotting assays.
Antibody generation
IsOATP4056- EL-6 polyclonal antibodies used in this study were generated as described36 at a commercial facility (GenScript, USA). The I. scapularis IsOATP4056 EL-6 epitope region was used for the generation of anti-EL6 antibody36.
Total RNA, DNA isolation and qRT-PCR and qPCR data analysis
Total RNA from ticks (unfed/fed) was extracted using Aurum total RNA mini kit (BioRad, USA) following manufacturer instructions37,47,48,49. The cDNA was generated from total RNA using iScript cDNA synthesis kit (BioRad, USA)37,47,48,49, and used as template for the amplification of H. longicornis oatps and actin. DNA from ticks was extracted using DNeasy blood and tissue kit (Qiagen, USA). Oligonucleotides for actin were used from published study50. All other oligonucleotides used in this study are mentioned in Supplementary Table 2. qPCR and qRT-PCR was performed using iQ-SYBR Green supermix (BioRad, USA) or 2× Universal SYBR Green fast qPCR mix (ABclonal, USA) and CFX96 touch system (BioRad, USA)37,47,48,49. As an internal control and to normalize the amount of template, tick actin amplicons were quantified. The standard curves were prepared using tenfold serial dilutions starting from 1 to 0.000001 ng/μl of known quantities of respective gene fragments.
Generation of A. phagocytophilum-infected H. longicornis nymphs
Unfed uninfected H. longicornis nymphs were bathed in 100 μl of A. phagocytophilum-DC preparation for 40 min at 34 °C. Ticks were washed three times with 1× PBS and stored in the tick environmental chamber for 1 week. After day 3, 5, 7 and 10 post infection, the nymphs were washed three times with 1× PBS, and the last wash of 1× PBS was saved as a negative control sample. Individual nymphs were processed for DNA extractions. DNA samples from the individual nymphs and the last 1× PBS wash sample were analyzed by qPCR to measure the bacterial loads.
Tick experiments with xanthurenic acid (XA)
Anaplasma phagocytophilum-infected H. longicornis nymphs (day 7 post-infected) were used in this experiment. Stock of XA (10 mM) (Sigma, USA) was made in 0.5 N NaOH solution34,37. A 1:10 dilution of the stock was prepared in 1X PBS to a final concentration of 1 mM and used in all experiments. A mock solution was prepared in a similar way but without XA. Anaplasma phagocytophilum-infected H. longicornis nymphs were bathed in 500 μl of 100 μM XA or mock solution for 40 min at 34 °C. Ticks were washed 3 times with 1× PBS and stored in the tick environmental chamber for 24 h. Individual nymph was then processed further for DNA or RNA extractions followed by qPCR or qRT-PCR analysis to measure bacterial burden or H. longicornis oatp transcripts, respectively.
Tick experiments with EL-6 antibody
Anaplasma phagocytophilum-infected H. longicornis nymphs were bathed in 500 μl of 1× PBS containing 5 μg of EL-6 antibody or control IgG antibody for 40 min at 34 °C. Ticks were washed 3 times with 1× PBS and stored in the tick environmental chamber for 24 h. Individual nymph was then processed further for DNA extractions followed by qRT-PCR analysis to measure the bacterial loads.
Western blotting assay
Immunoblotting was performed as described48. Five A. phagocytophilum-infected unfed H. longicornis and five A. phagocytophilum-infected unfed I. scapularis nymphs were crushed and homogenized using pellet pestle cordless motor (Biospec, OK) and pellet pestle (VWR, USA) in modified-RIPA lysis buffer supplemented with EDTA-free protease inhibitor cocktail to generate tick lysates. Protein concentrations were determined by Bradford (BCA) protein assay kit (Pierce, USA) and as per the manufacturer’s recommendations. Tick lysates (10 μg) were mixed with Laemmli sample buffer, boiled for 5 min, and resolved on 8% or 12% SDS-PAGE gel (in reducing and boiling or non-reducing and no boiling conditions). Gel was run at 110 V and stained with Ponceau-S dye, destained with water, and then transferred to nitrocellulose membrane. Ponceau-S-stained image served as loading control. Membrane was blocked with 5% BSA in 1× TBST (1X TBS, 0.05% Tween 20). EL-6 antibody was used at a dilution of 1:500 in 5% BSA in 1× TBST. HRP-conjugated goat anti-rabbit secondary antibody was used at a dilution of 1:5000 in 5% BSA in 1× TBST. Development of the chemiluminescent substrate was visualized using a BioRad ChemiDoc Touch Imaging System (BioRad, USA).
Sequence alignment and bioinformatic analysis
GenBank accession numbers for the sequences used in this study are mentioned in Supplementary Table 3. Amino acid sequence alignments for H. longicornis OATPs and orthologs from various organisms were performed using DNASTAR CLUSTALW alignment software. Sequence analyses were performed for H. longicornis OATPs with various organisms. The phylogenetic tree was constructed using the neighbor-joining method (BIONJ) using BIONJ algorithm in DNSTAR. The protein sequences for H. longicornis OATPs were downloaded from GenBank and individually analyzed at the PROSITE (http://prosite.expasy.org/) for the prediction of N-glycosylation, myristoylation, protein kinase C phosphorylation, casein kinase II phosphorylation and cAMP or c-GMP-dependent protein kinase phosphorylation sites and as described37,51,52. The number of post-translational modification sites in H. longicornis and I. scapularis are shown in Supplementary Table 4.
Statistical analysis
Statistical significance in the data sets was analyzed using GraphPad Prism6 software (https://www.graphpad.com/) and Microsoft Excel 2010 (https://www.microsoft.com). A student’s t-test was used to compare statistical significance between the groups, or ANOVA was used to compare the variations in the groups. P values of < 0.05 were considered significant in all analyses.
Ethics statement
Ixodes scapularis larvae feeding on mice was performed in accordance with University of Tennessee, Knoxville Institutional Animal Care and Use Committee (IACUC, animal assurance number: D16-00397) and approved protocol 2801-0221. The tick feeding experimental protocol was approved by the UTK, IACUC committee. The study was performed in accordance with ARRIVE guidelines. Acepromazine was used as a tranquilizer and was administered to animals prior to placement of larvae on mice and all efforts were made to minimize suffering.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
Jia, N. et al. Haemaphysalis longicornis. Trends Genet. 37, 292–293. https://doi.org/10.1016/j.tig.2020.11.008 (2021).
Hoogstraal, H., Roberts, F. H., Kohls, G. M. & Tipton, V. J. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol. 54, 1197–1213 (1968).
Wormser, G. P. et al. First recognized human bite in the United States by the Asian longhorned tick, Haemaphysalis longicornis. Clin. Infect. Dis. 70, 314–316. https://doi.org/10.1093/cid/ciz449 (2020).
Zhao, L. et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet Health 4, e320–e329. https://doi.org/10.1016/s2542-5196(20)30145-5 (2020).
Mahara, F. Japanese spotted fever: Report of 31 cases and review of the literature. Emerg. Infect. Dis. 3, 105–111. https://doi.org/10.3201/eid0302.970203 (1997).
Beard, C. B. et al. Multistate infestation with the exotic disease-vector tick Haemaphysalis longicornis—United States, August 2017–September 2018. MMWR Morb. Mortal. Wkly. Rep. 67, 1310–1313. https://doi.org/10.15585/mmwr.mm6747a3 (2018).
Rainey, T., Occi, J. L., Robbins, R. G. & Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey. US. J. Med. Entomol. 55, 757–759. https://doi.org/10.1093/jme/tjy006 (2018).
Price, K. J. et al. First detection of human pathogenic variant of Anaplasma phagocytophilum in field-collected Haemaphysalis longicornis, Pennsylvania, USA. Zoonoses Public Health 69, 143–148. https://doi.org/10.1111/zph.12901 (2022).
Eleftheriou, A., Beckett, J., Bai, N. & Pesapane, R. An established population of Asian longhorned ticks (Acari: Ixodidae) in Ohio, USA. J. Med. Entomol. https://doi.org/10.1093/jme/tjad104 (2023).
Price, K. J. et al. Evidence of protozoan and bacterial infection and co-infection and partial blood feeding in the invasive tick Haemaphysalis longicornis in Pennsylvania. J. Parasitol. 109, 265–273. https://doi.org/10.1645/22-122 (2023).
Lee, M. J. & Chae, J. S. Molecular detection of Ehrlichia chaffeensis and Anaplasma bovis in the salivary glands from Haemaphysalis longicornis ticks. Vector Borne Zoonotic Dis. 10, 411–413. https://doi.org/10.1089/vbz.2008.0215 (2010).
Meng, Z. et al. Detection of co-infection with Lyme spirochetes and spotted fever group rickettsiae in a group of Haemaphysalis longicornis. Zhonghua Liu Xing Bing Xue Za Zhi 29, 1217–1220 (2008).
Tufts, D. M. et al. Association of the invasive Haemaphysalis longicornis tick with vertebrate hosts, other native tick vectors, and tick-borne pathogens in New York City, USA. Int. J. Parasitol. 51, 149–157. https://doi.org/10.1016/j.ijpara.2020.08.008 (2021).
Rochlin, I. Modeling the Asian Longhorned Tick (Acari: Ixodidae) suitable habitat in North America. J. Med. Entomol. 56, 384–391. https://doi.org/10.1093/jme/tjy210 (2019).
Bakken, J. S. & Dumler, J. S. Human granulocytic anaplasmosis. Infect. Dis. Clin. N. Am. 29, 341–355. https://doi.org/10.1016/j.idc.2015.02.007 (2015).
Dumler, J. S. et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11, 1828–1834. https://doi.org/10.3201/eid1112.050898 (2005).
Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 24, 469–489. https://doi.org/10.1128/cmr.00064-10 (2011).
Zeman, P. et al. High seroprevalence of granulocytic ehrlichiosis distinguishes sheep that were the source of an alimentary epidemic of tick-borne encephalitis. Wien. Klin. Wochenschr. 116, 614–616. https://doi.org/10.1007/s00508-004-0191-0 (2004).
Anderson, J. F. & Magnarelli, L. A. Biology of ticks. Infect. Dis. Clin. N. Am. 22, 195–215 (2008).
Hodzic, E. et al. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36, 3574–3578. https://doi.org/10.1128/jcm.36.12.3574-3578.1998 (1998).
Carlyon, J. A. & Fikrig, E. Invasion and survival strategies of Anaplasma phagocytophilum. Cell Microbiol. 5, 743–754. https://doi.org/10.1046/j.1462-5822.2003.00323.x (2003).
Alberdi, P., Espinosa, P. J., Cabezas-Cruz, A. & de la Fuente, J. Anaplasma phagocytophilum manipulates host cell apoptosis by different mechanisms to establish infection. Vet. Sci. https://doi.org/10.3390/vetsci3030015 (2016).
Cabezas-Cruz, A., Espinosa, P., Alberdi, P. & de la Fuente, J. Tick-pathogen interactions: The metabolic perspective. Trends Parasitol. 35, 316–328. https://doi.org/10.1016/j.pt.2019.01.006 (2019).
Choi, K. S., Park, J. T. & Dumler, J. S. Anaplasma phagocytophilum delay of neutrophil apoptosis through the p38 mitogen-activated protein kinase signal pathway. Infect. Immun. 73, 8209–8218. https://doi.org/10.1128/IAI.73.12.8209-8218.2005 (2005).
Nelson, C. M. et al. Global transcription profiles of Anaplasma phagocytophilum at key stages of infection in tick and human cell lines and granulocytes. Front. Vet. Sci. 7, 111. https://doi.org/10.3389/fvets.2020.00111 (2020).
O’Neal, A. J., Singh, N., Mendes, M. T. & Pedra, J. H. F. The genus Anaplasma: Drawing back the curtain on tick-pathogen interactions. Pathog. Dis. https://doi.org/10.1093/femspd/ftab022 (2021).
Oliva Chavez, A. S. et al. An O-methyltransferase is required for infection of tick cells by Anaplasma phagocytophilum. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1005248 (2015).
Shaw, D. K. et al. Infection-derived lipids elicit an immune deficiency circuit in arthropods. Nat. Commun. 8, 14401. https://doi.org/10.1038/ncomms14401 (2017).
Hagenbuch, B. & Meier, P. J. Organic anion transporting polypeptides of the OATP/ SLC21 family: Phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 447, 653–665. https://doi.org/10.1007/s00424-003-1168-y (2004).
Kalliokoski, A. & Niemi, M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 158, 693–705. https://doi.org/10.1111/j.1476-5381.2009.00430.x (2009).
Nigam, S. K. et al. The organic anion transporter (OAT) family: A systems biology perspective. Physiol. Rev. 95, 83–123. https://doi.org/10.1152/physrev.00025.2013 (2015).
Stieger, B. & Hagenbuch, B. Organic anion-transporting polypeptides. Curr. Top Membr. 73, 205–232. https://doi.org/10.1016/b978-0-12-800223-0.00005-0 (2014).
Roth, M., Obaidat, A. & Hagenbuch, B. OATPs, OATs and OCTs: The organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br. J. Pharmacol. 165, 1260–1287. https://doi.org/10.1111/j.1476-5381.2011.01724.x (2012).
Dahmani, M., Anderson, J. F., Sultana, H. & Neelakanta, G. Rickettsial pathogen uses arthropod tryptophan pathway metabolites to evade reactive oxygen species in tick cells. Cell Microbiol. 22, e13237. https://doi.org/10.1111/cmi.13237 (2020).
Khanal, S., Taank, V., Anderson, J. F., Sultana, H. & Neelakanta, G. Arthropod transcriptional activator protein-1 (AP-1) aids tick-rickettsial pathogen survival in the cold. Sci. Rep. 8, 11409. https://doi.org/10.1038/s41598-018-29654-6 (2018).
Mahesh, P. P., Namjoshi, P., Sultana, H. & Neelakanta, G. Immunization against arthropod protein impairs transmission of rickettsial pathogen from ticks to the vertebrate host. NPJ Vaccines 8, 79. https://doi.org/10.1038/s41541-023-00678-y (2023).
Namjoshi, P., Dahmani, M., Sultana, H. & Neelakanta, G. Rickettsial pathogen inhibits tick cell death through tryptophan metabolite mediated activation of p38 MAP kinase. iScience 26, 105730. https://doi.org/10.1016/j.isci.2022.105730 (2023).
Neelakanta, G. & Sultana, H. Tick saliva and salivary glands: What do we know so far on their role in arthropod blood feeding and pathogen transmission. Front. Cell Infect. Microbiol. https://doi.org/10.3389/fcimb.2021.816547 (2021).
Ramasamy, E., Taank, V., Anderson, J. F., Sultana, H. & Neelakanta, G. Repression of tick microRNA-133 induces organic anion transporting polypeptide expression critical for Anaplasma phagocytophilum survival in the vector and transmission to the vertebrate host. PLoS Genet. https://doi.org/10.1371/journal.pgen.1008856 (2020).
Taank, V. et al. Human rickettsial pathogen modulates arthropod organic anion transporting polypeptide and tryptophan pathway for its survival in ticks. Sci. Rep. 7, 13256. https://doi.org/10.1038/s41598-017-13559-x (2017).
Taank, V. et al. Characterization of tick organic anion transporting polypeptides (OATPs) upon bacterial and viral infections. Parasit. Vectors 11, 593. https://doi.org/10.1186/s13071-018-3160-6 (2018).
Taank, V., Ramasamy, E., Sultana, H. & Neelakanta, G. An efficient microinjection method to generate human anaplasmosis agent Anaplasma phagocytophilum-infected ticks. Sci. Rep. 10, 15994. https://doi.org/10.1038/s41598-020-73061-9 (2020).
Radulovic, Z., Porter, L. M., Kim, T. K. & Mulenga, A. Comparative bioinformatics, temporal and spatial expression analyses of Ixodes scapularis organic anion transporting polypeptides. Ticks Tick-borne Dis. 5, 287–298. https://doi.org/10.1016/j.ttbdis.2013.12.002 (2014).
Mulenga, A., Khumthong, R., Chalaire, K. C., Strey, O. & Teel, P. Molecular and biological characterization of the Amblyomma americanum organic anion transporter polypeptide. J. Exp. Biol. 211, 3401–3408. https://doi.org/10.1242/jeb.022376 (2008).
Lee, W. et al. Polymorphisms in human organic anion-transporting polypeptide 1A2 (OATP1A2): Implications for altered drug disposition and central nervous system drug entry. J. Biol. Chem. 280, 9610–9617. https://doi.org/10.1074/jbc.M411092200 (2005).
Zhang, Y. C., Boxberger, K. H. & Hagenbuch, B. Organic anion transporting polypeptide 1B3 can form homo- and hetero-oligomers. PLoS One 12(6), e0180257 (2017).
Khanal, S., Taank, V., Anderson, J. F., Sultana, H. & Neelakanta, G. Rickettsial pathogen perturbs tick circadian gene to infect the vertebrate host. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23073545 (2022).
Sultana, H. et al. Anaplasma phagocytophilum induces actin phosphorylation to selectively regulate gene transcription in Ixodes scapularis ticks. J. Exp. Med. 207, 1727–1743. https://doi.org/10.1084/jem.20100276 (2010).
Turck, J. W., Taank, V., Neelakanta, G. & Sultana, H. Ixodes scapularis Src tyrosine kinase facilitates Anaplasma phagocytophilum survival in its arthropod vector. Ticks Tick-borne Dis. 10, 838–847. https://doi.org/10.1016/j.ttbdis.2019.04.002 (2019).
Hatta, T. et al. Leucine aminopeptidase in the ixodid tick Haemaphysalis longicornis: Endogenous expression profiles in midgut. J. Vet. Med. Sci. 71, 589–594. https://doi.org/10.1292/jvms.71.589 (2009).
Sultana, H., Patel, U., Toliver, M., Maggi, R. G. & Neelakanta, G. Molecular identification and bioinformatics analysis of a potential anti-vector vaccine candidate, 15-kDa salivary gland protein (Salp15), from Ixodes affinis ticks. Ticks Tick-borne Dis. 7, 46–53. https://doi.org/10.1016/j.ttbdis.2015.08.003 (2016).
Neelakanta, G., Sultana, H., Sonenshine, D. E. & Andersen, J. F. Identification and characterization of a histamine-binding lipocalin-like molecule from the relapsing fever tick Ornithodoros turicata. Insect Mol. Biol. 27, 177–187. https://doi.org/10.1111/imb.12362 (2018).
Acknowledgements
The following reagents were provided by Centers for Disease Control and Prevention for distribution by BEI Resources, NIAID, NIH: Ixodes scapularis larvae (live), NR-44115. The following reagent was deposited by the Centers for Disease Control and Prevention and obtained through BEI Resources, NIAID, NIH: Haemaphysalis longicornis larvae, NR-51847, nymph, NR-51848, adult, NR-51846. The following reagent was obtained through BEI Resources, NIAID, NIH: Anaplasma phagocytophilum, strain NCH-1, NR-48807. DML was supported by USDA FAS Faculty Exchange Program. This study was supported by funding in part from National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (Award number: R01AI130116) to GN and University of Tennessee, Knoxville Start-up funds to HS and GN.
Funding
This work was funded by National Institute of Allergy and Infectious Diseases, Grant No. R01AI130116.
Author information
Authors and Affiliations
Contributions
PN, DL, GN performed the experiments. PN, DL, HS and GN analyzed the data. PN, HS and GN designed the study, HS and GN provided reagents. All authors read, edited, and approved the manuscript. PN and GN wrote the paper. GN conceptualized and conceived the study and supervised overall investigations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Namjoshi, P., Lubembe, D.M., Sultana, H. et al. Antibody-blocking of a tick transporter impairs Anaplasma phagocytophilum colonization in Haemaphysalis longicornis ticks. Sci Rep 14, 9003 (2024). https://doi.org/10.1038/s41598-024-59315-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-59315-w
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.