Abstract
To evaluate association of vitamin D with sleep quality in adults and the influence of VDR-gene polymorphism FokI (rs2228570;A > G). Cross-sectional population-based study in adults, conducted in Brazil. The outcome was sleep-quality, evaluated by the Pittsburgh Sleep Quality Index. Vitamin D was determined by indirect electrochemiluminescence and classified as deficiency (VDD), 25(OH)D < 20 ng/mL in a healthy population or 25(OH)D < 30 ng/mL for groups at risk for VDD. FokI polymorphism in the VDR-gene was genotyped by qPCR and classified as homozygous wild (FF or AA), heterozygous (Ff or AG), or homozygous mutant (ff or GG). Multivariate logistic analysis was used to estimate the association between vitamin D and FokI polymorphism with sleep-quality. In a total of 1674 individuals evaluated, 53.6% had poor-sleep-quality, 31.5% had VDD, and the genotype frequency of the FokI polymorphism was 9.9% FF, 44.6% Ff, and 45.5% ff. In multivariate analysis, individuals with VDD had 1.51 times the chance of poor-sleep-quality, and individuals with the ff genotype had 1.49 times the chance of poor-sleep-quality (OR:1.49;95%CI:1.05–2.12) when compared to individuals with the FF or Ff genotype. In the combined analysis, individuals with VDD and ff genotype had more chance of poor-sleep-quality than individuals with sufficient vitamin D and genotype Ff or FF (OR:2.19;95%CI:1.27–3.76). Our data suggest that VDD and VDR FokI gene polymorphism are associated with poor-sleep-quality, and combining the two factors increases the chance of poor-sleep-quality compared to separate groups.
Similar content being viewed by others
Introduction
Vitamin D performs several functions, primarily by regulating osteomineral physiology, particularly calcium metabolism1. However, current evidence indicates that the pleiotropic effects of vitamin D and its metabolites extend beyond bone mineral metabolism and parathyroid gland activity, with products linked to other potential areas that are mainly sleep-related2,3. The precise causal mechanisms underlying this association remain elusive, but hypotheses have been proposed regarding the involvement of vitamin D receptors located intracellularly in areas of the brain responsible for regulating the sleep–wake cycle as well as the role of pro-inflammatory mediators4,5. Experimental evidence suggests an inverse relationship between sleep regulatory substance and vitamin D levels4. However, the biological effects of vitamin D rely on its binding to the vitamin D receptor (VDR), which interacts with other coregulatory proteins to modulate the transcription of target genes responsive to vitamin D. Consequently, variations in the VDR gene can influence the binding affinity of the receptor for vitamin D, thereby altering the response to this vitamin across different tissues6.
The FokI polymorphism (rs2228570) within the first exon has been extensively studied and characterized. This polymorphism results in a protein with reduced transcriptional activity and a diminished binding affinity for vitamin D7,8. Such alterations can affect the physiological responses to vitamin D in tissues, potentially affecting those associated with sleep regulation. Hence, it is plausible to hypothesize that this polymorphism in VDR may yield functional consequences similar to those observed in vitamin D deficiency, suggesting a potential link to poor sleep quality. Additionally, evidence suggests that this polymorphism may be associated with health outcomes that are intrinsically linked to sleep, such as major depressive disorder9, cognitive functioning10, and fibromyalgia11, with sleep possibly acting as a mediator of these relationships. Moreover, initial investigations have suggested a potential association between the FokI polymorphism and obstructive sleep apnea, a prominent sleep disorder12,13.
Therefore, we hypothesized that vitamin D deficiency and the presence of a homozygous polymorphic (ff or GG) genotype are positively associated with poor sleep quality. Therefore, this study aimed to evaluate the association between vitamin D deficiency, the VDR gene polymorphism FokI (rs2228570), and sleep quality.
Methods
Study design
This is a cross-sectional, population-based survey by multistage probability cluster sampling, conducted between October and December 2020 in two medium-sized cities (Ouro Preto and Mariana) in the south-central region of Minas Gerais, known as Iron Quadrangle, one of the largest iron ore producing areas in Brazil.
The data collection was carried out in the sample design in three steps: census sector, household, and resident, with the representativeness of the different socioeconomic strata (< 1 minimum wage, 1 to 3 minimum wages, and ≥ 4 minimum wages) guaranteed in the final sample.
The sample size was calculated using the OpenEpi tool (https://www.openepi.com/Menu/OE_Menu.htm), considering the prevalence of poor sleep quality considered as 50%, according to previous epidemiological surveys in adults, confidence level of 95%, design effect equal to 1.5 and precision of 5%. To ensure accuracy, the sample was increased by 20% to compensate for eventual losses due to refusals, the absence of the resident selected for the study, and closed households. Based on the sample calculation, 687 and 684 individuals should have been interviewed in Ouro Preto and Mariana, totaling 1371 individuals in the two cities evaluated. During the data collection process, we evaluated 1762 individuals, of which 88 were not analyzed due to insufficient blood samples for dosing of 25-hydroxyvitamin D and extraction of viable DNA for genotyping. Therefore, 1674 individuals representing adult residents in the urban areas of the two cities were included in this study. Face-to-face interviews were conducted in the resident’s homes using an electronic form by the interviewer. The questionnaire included sociodemographic and economic aspects, living habits, general health conditions, and sleep quality. All procedures were performed according to the Brazilian guidelines and standards for research involving human beings of the Declaration of Helsinki and were approved by the Research Ethics Committee (Ethics Submission Certificate no. 32815620.0.1001.5149). This study followed reported guidelines dictated by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). For more details on data collection, see Meireles et al.14.
Sleep quality
Sleep quality was evaluated by the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989). The Brazilian version of the PSQI had an overall reliability coefficient (Cronbach’s α) of 0.82, indicating a high degree of internal consistency15. This instrument is composed of 19 questions categorized into seven components, each component scoring from 0 to 3: subjective sleep quality (C1), sleep latency (C2), sleep duration (C3), habitual sleep efficiency (C4), sleep disturbances (C5), sleep medication use (C6), and daytime dysfunction (C7). The sum of the scores produces an overall score ranging from 0 to 21, where the highest score indicates poorer sleep quality16. In this study, sleep quality was classified as “good sleep quality” when the PSQI score was less or equal to 5 and as “poor sleep quality” when the PSQI was greater than 515,16.
Biological sample collection
Blood collection was performed by a trained professional by puncture in the region of the cubital fossa. For collection, a 7.5 mL S-Monovette® (Sarstedt) serum gel tube was used for vitamin D analysis; and a 2.7 mL S-Monovette® (Sarstedt) collection tube containing sodium fluoride/EDTA to obtain whole blood for molecular biology analyses. Subsequently, the samples were verified and transported to the Laboratory of Epidemiology (LEPI) of the School of Medicine at the Federal University of Ouro Preto (UFOP). In the laboratory, whole blood was stored in a freezer at − 20 °C, and the serum tubes were centrifuged at 2500 rpm for 15 min, and stored at − 80 °C for posterior analysis.
Vitamin D serum concentrations
Vitamin D was determined by indirect electrochemiluminescence with competition principle in the Access 2 Immunoassay System® (Beckman Coulter, USA) with a Roche Diagnostics® commercial kit (Roche, Switzerland). For intra-laboratory analysis, the coefficient of variation of the method ranges from 6.1 to 7.5%, and the correlation coefficient with LC–MS/M was 0.92 (data provided by the manufacturer). Vitamin D concentrations were classified as a deficiency when 25(OH)D < 20 ng/mL in a healthy population and 25(OH)D < 30 ng/mL for groups at risk for vitamin D deficiency (body mass index ≥ 30 kg/m2, age ≥ 60 years, individuals with brown or black skin color, pregnant women, and presence of cancer, diabetes or chronic kidney diseases)17.
VDR gene FokI polymorphism
The genomic DNA extraction was performed with Wizard® Genomic DNA Purification kit (Promega, USA) according to the manufacturer’s protocol. After extraction, the DNA was maintained for 24 h in a hydration solution and dosed by fluorimetry (Qubit 2.0 Fluorometer, Invitrogen®). The DNA samples were stored at -20 °C until use.
In this study, we evaluated the allelic discrimination of the FokI polymorphism (rs2228570 A > G) in the VDR gene, which consists of a nucleotide base change from adenosine (A) to guanine (G). The analysis was performed via the real-time PCR (qPCR) technique using the TaqMan® SNP Genotyping Assay System (Applied Biosystems, Foster City, USA) consisting of fluorescently labeled (FAM and VIC) probes (Applied Biosystems, Foster City, CA) in the 7500 Fast Real-Time PCR Systems equipment (Applied Biosystems, USA) (initial denaturation: 10 min, 95 ºC; ringing: 15 s, 40 cycles of 95 ºC; and final extension: 1 min, 60 ºC). Specific primers from Thermo Scientific (Assay ID__12060045_20) were used according to the manufacturer’s instructions.
Covariates
The questionnaire included variables for possible confounding controls in the association between vitamin D and sleep quality analysis5,18,19. The sociodemographic variables evaluated were sex (female or male), age group (18–34 years; 35–59 years; ≥ 60 years), marital status (single or married), current family income (≤ 2 minimum wages; > 2 to ≤ 4 minimum wages; > 4 minimum wages), and education level (< 8 years; 9–11 years; ≥ 12 years of study). Self-reported race/skin color was evaluated using the categories proposed by the Brazilian Institute of Geography and Statistics (IBGE) and were categorized into white, black, brown, and other races/skin colors (indigenous and yellows).
Health conditions evaluated were self-reported chronic diseases (hypertension, diabetes, asthma, lung disease, chronic kidney disease, cancer, heart or thyroid disease), which were dichotomized into morbidity (reporting at least one condition) and without morbidity (no disease). Furthermore, the following lifestyle variables were assessed: current smoking (yes or no), current alcohol drinking (yes or no), and physical activity (physically active when they reached at least 150 min of moderate-intensity aerobic physical activity or at least 75 min of vigorous-intensity aerobic physical activity per week, or physically inactive when the recommendations were not reached) (WHO, 2020). Nutritional status was evaluated by body mass index (BMI) from self-reported height (cm) and weight (kg). BMI was classified as underweight (BMI < 18.5 kg/m2 if aged < 60 years; BMI < 23.0 kg/m2 if old ≥ 60 years), eutrophic (BMI 18.5–24.9 kg/m2 if aged < 60 years; BMI 23.0–28.0 kg/m2 if aged ≥ 60 years), overweight (BMI 25.0–29.9 kg/m2 if old < 60 years; BMI 28.0–29.9 kg/m2 if aged ≥ 60 years), and obesity (BMI ≥ 30.0 kg/m2) according to WHO and PAHO for adults and elderly, respectively20,21
The anxiety and depression symptoms were evaluated by the Generalized Anxiety Disorder scale (GAD-7) and Patient Health Questionnaire (PHQ-9) scale, respectively22,23.For both scales, scores equal to or above 10 were considered to determine the presence of anxiety and depression symptoms22,23.
Daily sunlight was assessed quantitatively from the following questions: “From Monday to Sunday, how many times a week, and for how long are you exposed to the sun?” Subsequently, the average daily sunlight was calculated from the following formula: weekly frequency of sunlight (0–7 days) x daily time of sunlight (minutes)/7]. Moreover, we evaluated whether individuals used any vitamin D dietary supplements by self-reporting, “In the past three months, have you used a vitamin-based dietary supplement, such as vitamin D or cholecalciferol or cod oil supplementation?” (yes or no).
Statistical analysis
For statistical analyses, the Stata/MP program (version 15.0) was used with an alpha of 5%. All analyses were performed considering the study design and sampling weighting factors using the “svy” package. To characterize the sample, the distribution of continuous variables was assessed using the Shapiro–Wilk test, and all followed a parametric distribution. Subsequently, continuous variables were expressed as means and 95% confidence intervals (CI), and categorical variables were described as relative frequencies and 95% CI.
Allele frequencies were estimated using the gene counting method. Departure from Hardy–Weinberg equilibrium (HWE) was assessed via an exact two-sided probability test using the formula provided by Weir (1996).
A theoretical causality model based on a directed acyclic graph (DAG) was developed according to the exposure variables (vitamin D status and FokI polymorphism), outcome (sleep quality), and covariates using the online software Dagitty version 3.2. The causal connections, represented by arrows, were established between the variables (Fig. 1). Supplementary Table S1, “Health-related pathways overview illustrated in the directed acyclic graph (DAG),” provides a visual representation of the theoretical model developed for this study. To avoid unnecessary adjustments, spurious associations, and estimation errors, a backdoor criterion was used to select the minimum set of confounding variables required to fit the analyses. The model was adjusted using the following minimum and sufficient set of variables: age, sex, skin color, alcohol and tobacco consumption, body mass index, anxiety and depression symptoms, and exposure to sunlight.
Directed acyclic graph (DAG) on vitamin D and VDR FokI genotype with sleep quality in adults. The variable in green and with the “►” symbol inside the rectangle was the exposure variable; those in blue and with the “❙” symbol inside the rectangle was the outcome variable; variables represented in white circles represent variables not directly measured in the study; variables in green are the antecedents of the exposure variable; and those in red are antecedents of the outcome and exposure variables.
Therefore, vitamin D deficiency and FokI polymorphisms were evaluated independently and tested for their combined relationship with sleep quality. A combined interaction analysis was also performed. The variance inflation factor assessed collinearity between covariates with the “subsetByVIF” package considering a maximum cutoff point of 10 (VIF < 10).
Ethical considerations
All procedures followed the Brazilian standards for research involving human beings, the Declaration of Helsinki. This study was approved by the Research Ethics Committee of the Federal University of Minas Gerais (Ethics Submission Certificate No. 32815620.0.1001.5149) on September 22, 2020 (approval number: 4292475). Written informed consent was obtained from all participants.
Results
Characteristics of study participants
Table 1 shows the sociodemographic characteristics and health status of the study participants. Of the participants, 51.7% were female; the most prevalent age group was 35–59 years (45.4%); most were black, brown, or other skin colors (74.4%); married (53.5%); had 9–11 years of education (39.9%); and had a family income equal to or less than two minimum wages (45.1%). Supplementary Table S2 provides further insights into the sociodemographic and health conditions according to vitamin D levels, while Supplementary Table S3 presents similar information categorized by the FokI polymorphism.
Concerning health conditions, 31.5% of the individuals had vitamin D deficiency. The mean daily sunlight was 3.51 h; 6.5% used any vitamin D supplementation; 53.3% had poor sleep quality; 39.4% had chronic diseases; 57.7% and 17.0% consumed alcohol or tobacco, respectively; 36.8% and 18.6% were overweight or obese, respectively; and 23.4% and 15.8% had the presence of anxiety or depression symptoms, respectively (Table 1).
Distribution of FokI polymorphism
The VDR gene FokI polymorphism (rs2228570) genotype frequency was 9.9% (5.8–16.3) for wild homozygote (AA, also called FF), 44.6% (41.0–49.1) for heterozygote (AG also called Ff), and 45.5% (39.3–51.0) for mutated homozygote (GG also called ff) (Fig. 2). The distribution of genotypes for the FokI polymorphism did not deviate from the expectations predicted by HWE (p > 0.05), as determined using a chi-square test in both groups (X2:0,509; p-value: 0,475) (Fig. 2).
Distribution of VDR gene FokI polymorphism (rs2228570) genotype and allelic variants of the study population. Legend: HWE, Hardy–Weinberg equilibrium. Distributions of genotypes for FokI polymorphism did not deviate from expectations predicted by the HWE (p > 0.05) as determined by a chi-square test in both groups (X2: 0,509; p-value: 0,475).
Association of vitamin D and FokI polymorphism with sleep quality
Considering the vitamin D deficiency and FokI polymorphism as explanatory variables, individuals with vitamin D deficiency had a 1.55 chance of poor sleep quality (ORadjusted: 1.55; 95% CI: 1.01–2.40), and individuals with the ff genotype had a 1.49 chance of poor sleep quality (Oradjusted: 1.49; 95% CI: 1.04–2.15) compared to their counterparts (vitamin D sufficiency and FF or Ff genotype). In a combined analysis, individuals with vitamin D deficiency and ff genotype had a higher chance of poor sleep quality when compared to the isolated factors than individuals with sufficient vitamin D and Ff or FF genotype (ORadjusted: 2.19; 95% CI: 1.27–3.76) (Table 2).
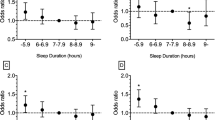
Furthermore, Fig. 3 shows the association of vitamin D and FokI polymorphisms with the moderate-to-difficult sleep subdomains of the PSQI. Therefore, we found that the combination of vitamin D deficiency and ff genotype was associated with poor subjective sleep quality (OR: 1.86; 95% CI: 1.07–3.16), shorter sleep duration (OR: 2.68; 95% CI: 1.19–6.27), greater sleep disturbances (OR: 2.10; 95% CI: 1.30–3.38), and more daytime dysfunction (OR: 2.15; 95% CI: 1.07–4.55).
Association of vitamin D deficiency and ff genotype FokI polymorphism with moderate to difficulties Pittsburgh Sleep Quality Index subdomains. Performed logistic regression adjusted by age, sex, skin color, alcohol and tobacco consumption, body mass index, physical activity, sunlight and mental health. The outcome variable was moderate to difficulty in the sleep domain, explanatory variable the individuals with vitamin D deficiency and ff genotype FokI polymorphism, and the reference group was vitamin D sufficiency and FF or Ff genotype FokI polymorphism. The score for each domain ranges from 0 to 3 (no difficulty to severe difficulty), and a domain score ≥ 2 indicates moderate to difficulty in the sleep domain.
Discussion
To the best of our knowledge, this is the first population-based study to evaluate the association between vitamin D and VDR gene FokI polymorphism (rs2228570) and poor sleep quality in adults. We verified that vitamin D deficiency and the ff genotype of the FokI polymorphism were associated with poor sleep quality, and the combined analysis of the two factors increased the risk of poor sleep quality compared with the separate groups.
There is evidence that sleep duration is shorter in adults, older individuals, and school-aged children5,18,24,25,26,27,28,29,30 when vitamin D levels are low. As demonstrated by Gao et al. (2018), in a systematic review and meta-analysis of 9397 participants, individuals with vitamin D deficiency had a significantly increased chance for sleep disturbances [OR: 1.50; (95% CI: 1.31–1.72)], poor sleep quality OR: 1.59; [(95% CI: 1.23–2.05)], short sleep duration OR: 1.74; (95% CI: 1.30–2.32)], and excessive sleepiness [OR: 1.36; (95% CI: 1.12–1.65)]2. These results are supported by other studies conducted in different populations5,18,24,25,30. This evidence comes from different methods of sleep assessment, such as objective measurement31, self-reporting32, and systematic and meta-analytic analyses2,33,34,35.
Although several studies have demonstrated this association, the main predictive factors for this relationship have not yet been fully identified. One of the proposed hypotheses suggests a possible link between the VDR and areas of the brain responsible for regulating sleep, such as the hypothalamus, as the VDR has already been identified in these regions36. Furthermore, Fang et al. (2020) demonstrated that melatonin can bind directly to the VDR, mainly in the ligand-binding domain located at the C-terminus of the VDR, thus influencing its transcriptional activity37. Consequently, VDR may serve as a nuclear target for melatonin, with the FokI polymorphism potentially altering the affinity and conformation of VDR, thereby affecting its interaction with melatonin and the subsequent effects on sleep regulation.
Therefore, the VDR modulates the biological actions of vitamin D in various tissues and organs38,39, including during sleep. In this regard, genetic alterations of the VDR, such as the FokI polymorphism, are objects of interest because they can modify the pathway of action of vitamin D, altering its interaction with target tissues40,41, and potentially being one of the possible causes of the relationship between vitamin D and sleep quality and sleep disorders12,42.
The FokI polymorphism is located in exon 2, the first translation initiation codon, and, to date, is the only variant identified to alter the protein structure of the VDR43. It is also considered an independent molecular marker because it does not appear to be in linkage disequilibrium with any other VDR gene polymorphisms44. When its polymorphic form is present, there is a change in the start codon position, indicating that the VDR protein expression is three amino acids longer than that of the wild-type allele. To fate, most studies have demonstrated that the shorter version of the protein (wild-type allele A or F, depending on the terminology used), containing 424 amino acids, is more active, in terms of its activity as a transcriptional factor45, than the long form (polymorphism-type allele G or f), which contains 427 amino acids. Jurutka et al.46 demonstrated that a VDR protein with 424 amino acids interacts more efficiently with transcription factors, resulting in a more active VDR protein46.
The influence of FokI polymorphisms on protein function and signaling remains poorly understood. Recent studies have demonstrated that the FokI polymorphism may be associated with a wide variety of pathological and physiological phenotypes in different populations, such as variations in 1,25(OH)2D3 concentration47, variations in bone mineral density and fractures 48, alterations in insulin secretion and sensitivity to action49, metabolic syndrome50, and increased risk of some cancers51, depression, schizophrenia, bipolar disorder, and seasonal affective disorder.
In the present study, the polymorphic ff genotype of VDR FokI was associated with poor sleep quality. Most sleep disorders result from complex interactions between environmental and social factors and individual genetic predispositions52. Regarding vitamin D-related polymorphisms, there are yet to be studies evaluating the relationship between FokI polymorphisms and sleep quality. Only two small studies have investigated the role of VDR polymorphisms in other sleep disorders. In the first study, Kirac et al.53 investigated two VDR gene polymorphisms (FokI and BsmI) in 50 individuals with obstructive sleep apnea (OSA) and 50 healthy individuals. They showed that VDR polymorphisms were significantly associated with OSA53. A second study conducted by Abbas et al. (2019) recruited 240 Egyptian adults with OSA and 120 matched controls and found a gradient dose–response, as one mutated allele (Ff) increased the risk of OSA by more than two times (OR: 2.66, p = 0.03), and when both mutated alleles were present (ff) the risk of OSA was 10 times higher (OR: 10.59, p < 0.001)12.
The role of vitamin D in the synthesis of two neurotransmitters, serotonin and melatonin, may explain this association. Serotonin also regulates mood, anxiety, and cognition. Melatonin controls the circadian rhythm of sleep-wakefulness. These neurotransmitters are derived from the amino acid tryptophan. The enzyme tryptophan hydroxylase 2, which converts tryptophan into serotonin, is activated by vitamin D. Furthermore, vitamin D suppresses the enzyme arylalcylamine N-acetyltransferase, which converts serotonin into melatonin54. This mechanism may be influenced by the FokI polymorphism in the VDR gene. The mutated allele of this gene may decrease the sensitivity to vitamin D and its effects on serotonin and melatonin synthesis. This can result in serotoninergic overactivity during the day and melatoninergic hypoactivity at night5. These changes can impair mood and sleep, increasing the risk of psychiatric disorders and sleep disturbance55. However, as shown in our study, vitamin D deficiency may worsen this condition as it decreases the availability of vitamin D to bind to the VDR and regulate serotonin and melatonin synthesis. In addition, the FokI polymorphism may upregulate the expression of vitamin D target genes in cells by modulating processes, such as cell differentiation, inflammation, and the circadian rhythm56. For example, body clock genes that control sleep–wake cycles can be regulated by vitamin D. Thus, individuals with the FF or Ff genotypes are more sensitive to the effects of vitamin D than those with the ff genotype.
Therefore, the combination of vitamin D deficiency and the FokI polymorphism may be associated with sleep, especially in individuals with a genetic or environmental predisposition to this condition. However, considering the limited number of studies that have evaluated the influence of the FokI polymorphism on sleep, we suggest that the ff genotype should be the focus of further studies to better understand its influence on poor sleep quality, confirm this hypothesis, and elucidate the causal mechanisms involved. Therefore, considering that more than half of the evaluated population had the ff genotype for the FokI polymorphism, vitamin D levels must be assessed and maintained at sufficient values to avoid compromising sleep quality. Furthermore, prevention and treatment strategies for sleep disorders that take into account the interactions between vitamin D and serotoninergic and melatoninergic neurotransmitters must be developed. These strategies include vitamin D supplementation, adequate sunlight or artificial light exposure, cognitive behavioral therapy, and case-specific pharmacological therapy. Thus, we aimed to improve the quality of life of people affected by these conditions.
Our study has several strengths. The sample design was robust to the following: (i) a representative random sample of the resident population from different socioeconomic strata; (ii) assessment by household survey; (iii) face-to-face study in a COVID-19 pandemic scenario; (iv) large sample size of genetic marker research; and (v) Use of DAG to guide analysis plans. Furthermore, it is essential to understand genetic variations in mixed populations (populations with two or more different ethnic origins) because these populations may inherit genetic factors that increase their susceptibility or resistance to chronic diseases, such as cancer, cardiovascular disease, diabetes, autoimmune diseases, and psychiatric disorders57. However, although our findings provide relevant insights, this study has limitations in some areas that deserve attention. First, the sleep quality information obtained was self-reported; therefore, an individual’s perception may be overestimated or underestimated compared to objective measures. However, the evaluation of sleep quality needs to be performed subjectively because it considers factors intrinsic to individuals’ perception of their sleep58,59,60. Furthermore, although we adjusted the models for potential confounders using the counterfactual approach of the DAG, residual confounding from the design method could not be excluded. Another limitation is the assessment of only the FokI polymorphism of the VDR gene, which may not fully capture the genetic variability of VDR and its implications in sleep regulation and vitamin D responses. Future investigations should consider analyzing other VDR gene polymorphisms, such as BsmI, ApaI, and TaqI, which may also contribute to these processes. However, our study is the first to establish a correlation between the VDR FokI polymorphism and sleep quality in a representative sample of the Brazilian adult population using a robust methodology (probabilistic sampling from a population-based household survey). This is significant because many existing studies are based on small, selected, or clinical samples, which can introduce selection bias and confounding factors.
Conclusion
This study suggests that the ff genotype of the VDR FokI (rs2228570) polymorphism is associated with poor sleep quality in adults. Furthermore, this genotype is associated with vitamin D deficiency, thereby increasing the risk of poor sleep. Therefore, considering that more than half of the evaluated population had the ff genotype of the FokI polymorphism, vitamin D levels must be assessed and maintained at sufficient levels to avoid sleep quality-related impairments.
Data availability
The data supporting the conclusions of this study can be obtained from the corresponding author on reasonable request.
References
Norman, A. W. From vitamin D to hormone D: Fundamentals of the vitamin D endocrine system essential for good health. Am. J. Clin. Nutr. https://doi.org/10.1093/AJCN/88.2.491S (2008).
Gao, Q. et al. The association between vitamin D deficiency and sleep disorders: A systematic review and meta-analysis. Nutrients 10(10), 1395. https://doi.org/10.3390/nu10101395 (2018).
Lai, Y. H. & Fang, T. C. The pleiotropic effect of vitamin D. ISRN Nephrol. 2013, 1–6. https://doi.org/10.5402/2013/898125 (2013).
Bellia, A. et al. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 8(1), 33–40. https://doi.org/10.1007/s11739-011-0559-x (2013).
Romano, F. et al. Vitamin D and sleep regulation: Is there a role for vitamin D?. Curr. Pharm. Des. 26(21), 2492–2496. https://doi.org/10.2174/1381612826666200310145935 (2020).
de Castro, L. C. G. O sistema endocrinológico vitamina D. Arq. Bras. Endocrinol. Metabol. 55(8), 566–575. https://doi.org/10.1590/S0004-27302011000800010 (2011).
Dastani, Z., Li, R. & Richards, B. Genetic regulation of vitamin D levels. Calcif. Tissue Int. 92(2), 106–117. https://doi.org/10.1007/s00223-012-9660-z (2013).
Neela, V. S. K. et al. Association of Taq I, Fok I and Apa I polymorphisms in vitamin D receptor (VDR) gene with leprosy. Hum. Immunol. 76(6), 402–405. https://doi.org/10.1016/j.humimm.2015.04.002 (2015).
Can, M. Ş, Baykan, H., Baykan, Ö., Erensoy, N. & Karlidere, T. Vitamin D levels and vitamin D receptor gene polymorphism in major depression. Psychiatr. Danub. 29(2), 179–185. https://doi.org/10.24869/PSYD.2017.179 (2017).
Kuningas, M. et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol. Aging 30(3), 466–473. https://doi.org/10.1016/J.NEUROBIOLAGING.2007.07.001 (2009).
Santos, S. K. F. S. et al. Evaluation of ApaI and FokI polymorphism of VDR gene and functional characterization in patients with fibromyalgia. Fisioter. Mov. 35, e35122. https://doi.org/10.1590/FM.2022.35122 (2022).
Abbas, A., Zayed, N. E., Abdel-Monem, M. & Abdelazem, A. S. Association of vitamin D receptor gene FokI polymorphism and serum vitamin D level in Egyptian patients with obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 68(2), 216–223. https://doi.org/10.4103/ejcdt.ejcdt (2019).
Ragia, G. et al. Genetics of obstructive sleep apnea: Vitamin D receptor gene variation affects both vitamin D serum concentration and disease susceptibility. OMICS 23(1), 45–53. https://doi.org/10.1089/omi.2018.0184 (2019).
Meireles, A. L. et al. COVID-Inconfidentes—SARS-CoV-2 seroprevalence in two Brazilian urban areas during the pandemic first wave: Study protocol and initial results. Poblac. Salud Mesoam. https://doi.org/10.15517/psm.v21i1.53127 (2023).
Bertolazi, A. N. et al. Validation of the Brazilian portuguese version of the Pittsburgh sleep quality index. Sleep Med. 12(1), 70–75. https://doi.org/10.1016/j.sleep.2010.04.020 (2011).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4 (1989).
de Moraes, A. C. F. et al. Posicionamento oficial da sociedade brasileira de patologia clínica/medicina laboratorial e da sociedade brasileira de endocrinologia e metabologia. J. Bras. Patol Med. Lab. 53(6), 377–381 (2018).
McCarty, D. E., Chesson, A. L., Jain, S. K. & Marino, A. A. The link between vitamin D metabolism and sleep medicine. Sleep Med. Rev. 18(4), 311–319. https://doi.org/10.1016/j.smrv.2013.07.001 (2014).
Muscogiuri, G. et al. The lullaby of the sun: The role of vitamin D in sleep disturbance. Sleep Med. 54, 262–265. https://doi.org/10.1016/j.sleep.2018.10.033 (2019).
PAHO PAHO. XXXVI Reunión Del Comitê Asesor de Investigaciones En Salud. (2001).
WHO. Physical Status: The Use and Interpretation of Anthropometry. Technical Report Series no 854. (1995).
Kroenke, K., Spitzer, R. L. & Williams, J. B. W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 16(9), 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x (2001).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166(10), 1092–1097. https://doi.org/10.1001/archinte.166.10.1092 (2006).
Menezes-Júnior, L. A. A. et al. Influence of sunlight on the association between 25-hydroxyvitamin D levels and sleep quality in Brazilian adults: A population-based study. Nutrition https://doi.org/10.1016/j.nut.2023.112008 (2023).
Menezes-Júnior, L. A. A. et al. Hypovitaminosis D is associated with sleep disorders in workers on alternating shifts with cardiovascular risk factors. medrxiv https://doi.org/10.1101/2021.05.04.21256625 (2021).
Gominak, S. C. & Stumpf, W. E. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med. Hypotheses 79(2), 132–135. https://doi.org/10.1016/j.mehy.2012.03.031 (2012).
Gong, Q. H. et al. 25-Hydroxyvitamin D status and its association with sleep duration in Chinese schoolchildren. Nutrients 10(8), 1013. https://doi.org/10.3390/nu10081013 (2018).
Lee, H. J., Choi, H. & Yoon, I. Y. Impacts of serum vitamin D levels on sleep and daytime sleepiness according to working conditions. J. Clin. Sleep Med. 16(7), 1045–1054. https://doi.org/10.5664/jcsm.8390 (2020).
Mosavat, M., Smyth, A., Arabiat, D. & Whitehead, L. Vitamin D and sleep duration: Is there a bidirectional relationship?. Horm. Mol. Biol. Clin. Investig. https://doi.org/10.1515/hmbci-2020-0025 (2020).
Neighbors, C. L. P. et al. Vitamin D and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. 43, 100–108. https://doi.org/10.1016/j.sleep.2017.10.016 (2018).
Piovezan, R. D. et al. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS One 12(7), 1–11. https://doi.org/10.1371/journal.pone.0180901 (2017).
Jung, Y. S. et al. The relationship between serum vitamin D levels and sleep quality in fixed day indoor field workers in the electronics manufacturing industry in Korea. Ann. Occup. Environ. Med. 29(1), 25. https://doi.org/10.1186/s40557-017-0187-7 (2017).
Abboud, M. & Vitamin, D. Supplementation and sleep: A systematic review and meta-analysis of intervention studies. Nutrients 14(5), 1076. https://doi.org/10.3390/NU14051076/S1 (2022).
Li, X., He, J. & Yun, J. The association between serum vitamin D and obstructive sleep apnea: An updated meta-analysis. Respir. Res. 21(1), 1–12. https://doi.org/10.1186/S12931-020-01554-2 (2020).
Mirzaei-Azandaryani, Z., Abdolalipour, S. & Mirghafourvand, M. The effect of vitamin D on sleep quality: A systematic review and meta-analysis. Nutr. Health https://doi.org/10.1177/02601060221082367 (2022).
Su, H., Liu, N., Zhang, Y. & Kong, J. Vitamin D/VDR regulates peripheral energy homeostasis via central renin-angiotensin system. J. Adv. Res. 33, 69. https://doi.org/10.1016/J.JARE.2021.01.011 (2021).
Fang, N. et al. Identification of a novel melatonin-binding nuclear receptor: Vitamin D receptor. J. Pineal Res. 68(1), e12618. https://doi.org/10.1111/jpi.12618 (2020).
Haussler, M. R. et al. Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 92(2), 77–98. https://doi.org/10.1007/s00223-012-9619-0 (2013).
Muszkat, P., Camargo, M. B. R., Griz, L. H. M. & Lazaretti-Castro, M. Evidence-based non-skeletal actions of vitamin D. Arq. Bras. Endocrinol. Metabol. 54(2), 110–117. https://doi.org/10.1590/s0004-27302010000200005 (2010).
Pike, J. W., Meyer, M. B., Lee, S. M., Onal, M. & Benkusky, N. A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 127(4), 1146. https://doi.org/10.1172/JCI88887 (2017).
Ryan, J. W., Anderson, P. H. & Morris, H. A. 2015 Pleiotropic activities of vitamin D receptors—adequate activation for multiple health outcomes. Clin. Biochem. Rev. 36(2), 53 (2015).
Baik, I., Seo, H. S., Yoon, D., Kim, S. H. & Shin, C. Associations of sleep apnea, NRG1 polymorphisms, alcohol consumption, and cerebral white matter hyperintensities: Analysis with genome-wide association data. Sleep 38(7), 1137–1143. https://doi.org/10.5665/sleep.4830 (2015).
Mahjoubi, I. et al. Lack of association between FokI polymorphism in vitamin D receptor gene (VDR) & type 2 diabetes mellitus in the Tunisian population. Indian J. Med. Res. 144(1), 46. https://doi.org/10.4103/0971-5916.193282 (2016).
Nejentsev, S. et al. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum. Mol. Genet. 13(15), 1633–1639. https://doi.org/10.1093/hmg/ddh169 (2004).
van Etten, E. et al. The vitamin D receptor gene FokI polymorphism: Functional impact on the immune system. Eur. J. Immunol. 37(2), 395–405. https://doi.org/10.1002/EJI.200636043 (2007).
Jurutka, P. W. et al. The Polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol. Endocrinol. 14(3), 401–420. https://doi.org/10.1210/mend.14.3.0435 (2000).
de Oliveira, A. C. R. et al. BsmI polymorphism in the vitamin D receptor gene is associated with 25-hydroxy vitamin D levels in individuals with cognitive decline. Arq. Neuropsiquiatr. 76(11), 760–766. https://doi.org/10.1590/0004-282x20180116 (2018).
Arai, H. et al. A vitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J. Bone Miner. Res. 12(6), 915–921. https://doi.org/10.1359/jbmr.1997.12.6.915 (1997).
Zakaria, W. N. A. et al. Association between vitamin D receptor polymorphisms (Bsmi and Foki) and glycemic control among patients with type 2 diabetes. Int. J. Environ. Res. Public Health 18(4), 1–18. https://doi.org/10.3390/ijerph18041595 (2021).
Schuch, N. J., Garcia, V. C., Vívolo, S. R. G. F. & Martini, L. A. Relationship between vitamin D receptor gene polymorphisms and the components of metabolic syndrome. Nutr. J. 12(1), 96. https://doi.org/10.1186/1475-2891-12-96 (2013).
Rukin, N. J. & Strange, R. C. What are the frequency, distribution, and functional effects of vitamin D receptor polymorphisms as related to cancer risk?. Nutr. Rev. 65, S96–S101. https://doi.org/10.1111/j.1753-4887.2007.tb00350.x (2008).
Bidaki, R., Zarei, M., Toosi, A. K. & Shooshtari, M. H. A review on genetics of sleep disorders. Iran J. Psychiatry Behav. Sci. 6(1), 12 (2012).
Kirac, D. et al. Different VDR, VDBP genotypes and vitamin D levels may effect obstructive sleep apnea syndrome. Cell. Mol. Biol. (Noisy-le-grand) 65(1), 46–51 (2019).
Lucock, M. et al. Vitamin D: Beyond metabolism. J. Evid. Based Complement. Altern. Med. 20(4), 310–322. https://doi.org/10.1177/2156587215580491 (2015).
Huiberts, L. M. & Smolders, K. C. H. J. Effects of vitamin D on mood and sleep in the healthy population: Interpretations from the serotonergic pathway. Sleep Med. Rev. 55, 101379. https://doi.org/10.1016/j.smrv.2020.101379 (2021).
Angel, B., Lera, L., Sánchez, H., Oyarzún, A. & Albala, C. FokI polymorphism in vitamin D receptor gene: Differential expression of TNFα in peripheral mononuclear cells of type 2 diabetic subjects. Meta Gene 7, 1–6. https://doi.org/10.1016/J.MGENE.2015.10.003 (2015).
Sul, J. H., Martin, L. S. & Eskin, E. Population structure in genetic studies: Confounding factors and mixed models. PLoS Genet. 14(12), e1007309. https://doi.org/10.1371/journal.pgen.1007309 (2018).
Fabbri, M. et al. Measuring subjective sleep quality: A review. Int. J. Environ. Res. Public Health 18(3), 1082. https://doi.org/10.3390/ijerph18031082 (2021).
Ibáñez, V., Silva, J. & Cauli, O. A survey on sleep questionnaires and diaries. Sleep Med. 42, 90–96. https://doi.org/10.1016/j.sleep.2017.08.026 (2018).
Ibáñez, V., Silva, J. & Cauli, O. A survey on sleep assessment methods. PeerJ https://doi.org/10.7717/PEERJ.4849 (2018).
Acknowledgements
The authors acknowledge the support of the Federal University of Ouro Preto (UFOP) and the Research and Education Group in Nutrition and Collective Health (GPENSC) for their support and incentive, Clinical Analysis Pilot Laboratory (LAPAC) of the Pharmacy School and Laboratory of Epidemiology (LEPI) of the School of Medicine at the Federal University of Ouro Preto (UFOP) for the biochemical analyses, and also, the support of the Municipal Health Secretariats of the municipalities.
Funding
This study was supported by the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) nº001/2021; APQ-02445-21], Brazilian Council for Scientific and Technological Development (CNPq), and Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES) 9/2020; nº88887.504994/2020e00] with finance code 001 for Ph.D. student scholarship.
Author information
Authors and Affiliations
Contributions
Luiz Antônio Alves de Menezes-Júnior—data collection, conception and study design; analysis and interpretation of data; writing the manuscript, critical review and final approval. Thaís S. Sabião—data collection, assistance in laboratory analysis, critical review and final approval. Samara Silva de Moura—data collection, assistance in laboratory analysis, critical review and final approval. Aline Priscila Batista—data collection, assistance in laboratory analysis, critical review and final approval. Mariana Carvalho de Menezes—project conception, management of financial resources, critical review and final approval. Júlia C. C. Carraro—conception and study design, interpretation of data; critical review, supervision and final approval. George L. L. Machado-Coelho—conception and coordination of data collection, management of financial, critical review, supervision and final approval. Adriana L. Meireles—conception and coordination of data collection, management of financial resources, interpretation of data, critical review and final approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Menezes-Júnior, L.A.A.d., Sabião, T.d.S., Moura, S.S.d. et al. The role of interaction between vitamin D and VDR FokI gene polymorphism (rs2228570) in sleep quality of adults. Sci Rep 14, 8141 (2024). https://doi.org/10.1038/s41598-024-58561-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-58561-2
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.