Abstract
Untreated HCV mono and HCV/HIV coinfected women have lower degrees of liver fibrosis (LF) compared to men. Direct acting antiviral (DAA) therapy attains viral eradication in > 90% of patients with progressive LF decline in parallel. Gender-related differences in LF regression in the long term assessed by non-invasive liver fibrosis markers (NILFM) in HCV mono and HCV/HIV coinfected after DAA treatment have not been explored so far. 374 HCV-infected adult patients, 214 of them HCV/HIV coinfected, were followed-up for 24 months after starting DAA therapy. LF was assessed by NILFM: transient elastometry (TE) and several biochemical indexes (APRI, Forns, FIB-4). Men had significantly more advanced LF at baseline than women assessed by NILFM. No LF differences at baseline in age, HIV coinfection course (CD4, HIV viral load), and HCV features (HCV viral load, genotype) were detected. No significant gender differences in LF decline after comparing 24-month and baseline LF values were observed. LF changes after DAA therapy were similar in HCV mono and HCV/HIV coinfected patients and in both sexes. Gender did not influence the course of LF decline after DAA assessed by NILFM: TE (P = 0.8), APRI (P = 0.9), Forns (P = 0.4) and FIB-4 (P = 0.7) by multivariate analysis. No gender differences in the 24 month LF decline after DAA with independence of having HCV mono or HCV/HIV coinfection were found.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) infection is a major cause of progressive liver fibrosis (LF) with cirrhosis, liver transplantation and hepatocarcinoma as its late-stage most severe complications1. Gender differences in LF in HCV-infected patients have been rarely explored in spite of the nowadays availability of non-invasive liver fibrosis markers (NILFM) including transient elastometry (TE), and biochemical indexes (APRI, Forns, FIB-4) instead of liver biopsy to assess LF2,3,4,5. Hormonal changes are involved in LF progression in HCV-infected women. Menopause is associated with accelerated LF while pregnancies and estrogen administration are LF-protective6,7,8. A protective effect of female gender on LF, lost after menopausal was reported in Chinese women with chronic hepatitis B infection9 On the other hand higher degrees of LF in men are associated with lower free testosterone and higher sex-hormone-binding globulin (SHBG)10. Cell-intrinsic androgen receptors could drive CD8+ T cell dysfunction and secondarily modulate LF in men as they do in cancer immunotherapy11. Different behaviors of men and women including the abuse of alcohol, tobacco or intravenous drugs, more prevalent among male patients, might enhance their HCV-induced LF. HIV infection could contribute to increasing LF in HCV/HIV-coinfected individuals12,13,14,15,16,17. However HCV/HIV coinfected women have lower degrees of LF compared to men with independence of their alcohol consumption18. Direct-acting antiviral agents (DAA) are extremely effective in combination and even in monotherapy to treat HCV infection with > 90% efficacy19. The dynamics of LF at long term after DAA are not well understood due to the lack of extended follow-up studies and the different tools used for LF assessment2,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24. It seems that LF decreases quickly after starting DAA therapy and then a slow decline or even a plateau in the LF curve is reached. The quick LF decrease has been attributed to early resolution of liver inflammation, although it does not always occur21. In 2022 we reported a continuous improvement of LF measured by NILFM in HCV mono and HCV/HIC coinfected patients after DAA therapy and 24 months of follow-up25. However in 2023 Gardner et al. observed a lack of decline beyond one year after HCV cure in LF assessed by an enhanced liver fibrosis (ELF) score, APRI and FIB-4 indexes in HCV/HIV coinfected American women26.
To our knowledge, no study to date has focused on the evaluation of fibrosis regression according to gender. We hypothesized that because HCV and HCV/HIV coinfected women had lower LF before DAA therapy compared to men, their LF reversal after DAA treatment might be different. This LF regression differences might be more evident after a long term follow-up. On the other hand, the presence of HCV mono or HCV/HIV coinfection could induce different degrees of LF regression.
The aim of this study was to comparatively evaluate the influence of gender in the long-term course of LF decline, as measured by NILFM, in HCV mono- and HCV/HIV coinfected patients treated with different DAA regimens, and to identify other factors that might modify LF regression after DAA therapy such as HIV coinfection. To this end we have carried out a prospective and comprehensive study on a large sample of HCV mono- and HCV/HIV coinfected male and female Spanish patients who were followed-up at regular intervals for 24 months after DAA treatment.
Patients and methods
Patients
Patients with active HCV monoinfection or HCV-HIV coinfection demonstrated by positive serology and viral RNA plasma levels were enrolled in the study when starting DAA therapy. Patients were Caucasians, older than 18 years and were recruited from three third level hospitals of Northwest Spain. A number of demographic, epidemiological, laboratory and clinical data were obtained from the patients and from their electronic medical charts. All HCV-HIV-coinfected patients were receiving ART at the inclusion time. The DAA regimens used included NS5B inhibitors (sofosbuvir, dasabuvir), NS3/4A inhibitors (glecaprevir, paritaprevir, asunaprevir, grazoprevir, simeprevir) and NS5A inhibitors (velpatasvir, ledipasvir, daclatasvir, pibrentasvir, ombistavir, elbasvir) with or without ribavirin. DAA were selected according to the attending clinician criteria. LF was assessed by TE (Fibroscan) and by the noninvasive biochemical biomarkers APRI, Forns and FIB-4 at baseline and at the 1st, 3rd, 6th, 12th and 24th months.
Exclusion criteria
To avoid LF confounding factors different from HCV and HIV infections, patients with HBV coinfection with/out delta virus coinfection, ethanol consumption ≥ 50 g/day for > 5 years, alcoholic hepatopathy, and other liver diseases were excluded from the study as we previously did27. Pregnant women and those individuals in whom there were technical difficulties for obtaining reliable TE readings were also excluded. In addition, patients with ascites or spontaneous bacterial peritonitis were excluded because TE reading could be altered by these factors5,28,29.
Transient elastometry
LF was assessed by TE using Fibroscan (EchoSens,Paris, France) following pre-established methods5,28,29. Patients were classified into 4 groups following the TE assessments (F0-F1, F2, F3 and F4), reflecting the progressive degree of LF by similarity with the histological stages of the METAVIR grading system. Thus, < 7.2 kPa measurements were considered minimal or no fibrosis (F0-F1), values in the range of 7.2–9.3 kPa as significant fibrosis (F2), those in the range of 9.4–13.9 kPa as advanced fibrosis (F3), and values > 13.9 kPa as cirrhosis (F4)25,28.
Laboratory methods
HIV and HCV serologies were assessed by enzyme immunoassay (MEIA AxSYM; Abbott Diagnostics,Abbott Park, IL, United States). HIV and HCV RNA by quantitative PCR (Cobas TaqMan; Roche Diagnostics, Branchburg, NJ, United States) and HCV genotypes by a line probe assay (Versant HCV, Siemens). Routine laboratory methods were used to calculate three LF indexes: AST and platelets for APRI index30, age, platelet counts, total cholesterol and GGT for Forns index31, and age, AST, ALT and platelet counts for FIB-432.
Statistical analysis
Due to the non-Gaussian distribution of the fibrosis markers studied, they underwent natural logarithmic transformation for analysis, and were back-transformed to the original units for reporting. Categorical variables are reported as percentage, continuous variables as mean and 95% confidence interval (CI), and the differences between sexes were assessed by the chi-square test and the t-test, respectively. Paired t-tests were used to compare the changes in the four NILFM between two time points within the same individual. The overall absolute and relative improvements in fibrosis indexes at the end of the follow-up period were calculated by subtracting and dividing, respectively, the values observed at the 24-month respect to the baseline measurements. A multivariate general linear model was elaborated for each of the four fibrosis markers, in order to compare their courses over time to verify if the behavior of these markers was influenced by gender. Statistical calculations were performed with the SPSS software v.25 (IBM Corp., Armonk, NY, USA). The cut-off for statistical significance was established at P < 0.05 for a two-sided test.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Hospital Universitario Central de Asturias (HUCA).
Consent to participate
All patients underwent standard of care, including routine noninvasive procedures. Informed consent was obtained from all individual participants included in the study.
Results
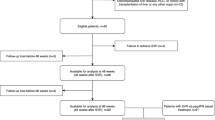
The study included 374 HCV-infected patients, 264 men and 110 women, who underwent DAA therapy and were followed-up for 24 months afterwards, although a total of 85 patients did not accomplish the last evaluation (25.4% of the women and 21.6% of the men). The mean age was 50.8 years (95% CI 50.0–51.6), 75.6% had genotype 1 infection and 57.2% were coinfected with HIV.
Table 1 shows the baseline characteristics of men and women, as well as the absolute and relative changes of the four NILFM at 24 months in comparison with the baseline values. There were no differences in age, HIV coinfection, HCV features and HCV treatment between the two groups. On the contrary, men had significantly higher levels of certain laboratory parameters, such as hemoglobin, γ-glutamyl transferase, bilirubin, creatinine, as well as lower levels of platelets, and total and HDL cholesterol than women.
Regarding LF issues, men had significantly more advanced liver disease at baseline than women, as evaluated by the non-invasive markers, but there were not significant differences in the absolute or relative changes of these markers when the 24th-month was compared with the baseline values.
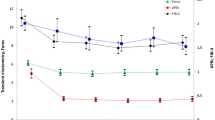
Figure 1 shows the course over time of the four non-invasive indexes studied. The baseline differences between men and women were maintained during follow-up, depicting therefore parallel curves. The figure also shows a downward course over time, which resulted in statistically significant differences between the intra-subject final and initial measurements for each marker in both sexes (P < 0.05 for each comparison). Therefore, men and women had a favorable and similar short and long term fibrosis responses to DAA therapy in univariate analysis.
Figure 2 shows the relative improvement in fibrosis markers at 24 months vs the initial measurements according to the baseline fibrosis stage. It can be appreciated that the degree of improvement was directly related to the baseline degree of fibrosis, experiencing higher reductions in fibrosis those patients with more advanced liver disease. Likewise, there were no significant differences between sexes, indicating similar fibrosis improvements in men and women regardless of the fibrosis stage.
Figure 3 depicts the relative improvements respect to the baseline values of the four markers in men and women according to the presence or absence of HIV co-infection. The fibrosis changes after DAA therapy were similar in monoinfected and in coinfected patients, and were also similar in both sexes for all markers (P = 0.08 to P = 0.9), with the only exception of TE in HCV/HIV coinfected patients, as women had somewhat lower improvements than men (difference between the means 14.4%, P = 0.03).
Finally, multivariate general linear models were constructed to evaluate the course over time of the four non-invasive markers of fibrosis, including variables that potentially could influence the degree and evolution of fibrosis, such as age, HIV infection, type, duration and virological results of DAA therapy, HCV viral load, and baseline fibrosis stage. Similarly to the univariate analysis described in Fig. 1, the downward course of the curves of men and women generated by the multivariate model were markedly parallel for all markers, indicating that gender did not influence the course of fibrosis as evaluated by these four markers: TE (P = 0.8), APRI (P = 0.9), Forns (P = 0.4), and FIB-4 (P = 0.7).
Discussion
Our study showed a continuous decline in the long term in LF assessed by 4 NILFM (TE, APRI, Forns, FIB-4) in HCV infected patients after DAA therapy with parallel LF curves in men and women and no gender differences. Men had significantly more advanced LF at baseline compared to women. However fibrosis improvement curves went in parallel in both sexes throughout the 24 month follow up with independence of the baseline fibrosis stage. No differences among sexes in the comparison of LF decline course after DAA treatment between HCV/HIV coinfected individuals were detected either. Our results suggest that gender does not play any significant role in the evolution of LF after DAA therapy in HCV-mono and HCV/HIV coinfected individuals.
We confirmed previous reports that HCV-monoinfected and HCV/HIV-coinfected male had higher LF at baseline compared to women14,18. Previous works have suggested a protective effect of estrogens on fibrogenesis by the inhibition of stellate cells33,34. Furthermore, low plasma estrone levels associated with increased disease severity in women with HCV-related liver cirrhosis35. The mean age of the women included in our study, 51.63 years, implied that they were already postmenopausal or near menopause and therefore with low estrogens plasma levels. However, any possible relationship between LF and hormone levels should be cautious, taking into account the substantial differences in sexual hormone levels between men and women and, even within each gender, the marked hormonal changes related to age, menopause, phase of menstrual cycle and severity of liver fibrosis. Females are more vulnerable to ethanol compared to male because their smaller volume of distribution and reduced gastric alcohol dehydrogenase activity36. However the increased LF we observed in men was independent of ethanol abuse, usually more prevalent in male, because patients with ethanol consumption ≥ 50 g/day for > 5 years were excluded from the study. Although it could be arguable the use of the same cut-off in men and women, no specific cut-off has been reliably defined for women and, in addition, alcohol did not seem to play any relevant deleterious role in women according to our observations, as the fibrosis course was similar and parallel from different perspectives, and men had baseline worse fibrosis values than women.
This men-associated LF enhancing effect was also independent of the presence of HIV-coinfection, and of its course assessed by CD4 levels and HIV viral load. Although we recorded data about HIV therapy, the multiplicity of drugs and regimens, both at baseline and during follow-up, as well as the fact that treatment regimens were composed of more than one drug, preclude the analysis of HIV-related drugs. In addition, it was independent of the HCV features assessed by HCV viral load and genotype.
Very few studies have focused in the LF improvement assessment in HCV patients after DAA treatment with an extended follow-up. Even more rarely these studies have focused on HCV-infected women mostly because drug abuse, the most current HCV transmission mechanism in developed countries, is less prevalent in women. However, all the HCV-infected women and men included in our study were former IV drug users. Gardner et al. report an early steep decline in LF measured by biochemical fibrosis markers (ELF, APRI, FIB-4) followed by a surprising flattening of the LF decline in 116 HCV/HIV-infected American women 1–2 years after HCV therapy26. This long-term flattening curve observation was more evident for ELF score than for the other fibrosis biomarkers. ELF score is a marker of extracellular modeling used instead of TE in Gardner´s work. ELF score is derived from hyaluronic acid, amino-terminal propeptide of type III collagen (PIIINP) and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1). The authors argued that were was little LF regression at long-term after HCV-therapy or that ELF score gave not an ideal reflection of fibrosis but rather liver inflammation26. We observed a continuous progressive LF curve decline in our study. However we assessed LF with TE, APRI, Forns and FIB-4 but not with the ELF score. Anyhow in a previous paper derived from the same study we observed a progressive decline in TIMP-1 plasma levels, a biochemical marker included in the ELF score after 2 year follow-up in HCV and HCV/HIC coinfected patients treated with DAA25. On the other hand, the findings of Gardner et al.26 about the improvement in APRI and FIB-4 markers within one year post-therapy, but not in the second year, may be explained and complemented by our findings based on shorter interval determinations. Thus, we observed a marked improvement in APRI and FIB-4 indexes during the first month of therapy, reaching a plateau afterwards. As AST and ALT are used for calculations of these indexes, an early and marked improvement in inflammation would be responsible for these observations. In fact, we analyzed the course of AST and ALT over time and, as expected, we found a marked decrease during the first month of therapy, averaging normal levels at this time point, followed by a flattening during the remaining follow-up (data not shown). These findings support the role of rapidly decreasing inflammation after the onset of therapy as the explanation of the early improvement, and further flattening, in these markers.
The strongest points of our study include its prospective nature, the very long follow-up after DAA therapy, the high number of patients enrolled, including 110 women, and the comprehensive evaluation at each time point. However there are also some limitations, including the lack of liver biopsies, the gold standard for fibrosis assessment. However, biopsies have several limitations including sampling error, cost, and risk of complications. Therefore liver biopsies are being replaced by TE, a technique we used along with other noninvasive biochemical markers of LF. Also, sexual hormone determinations were beyond the purpose of the study and were not available, but their interpretation would be difficult taking into account the large differences between men and women, and the great variability in their serum levels commented above, which make very difficult the drawing of reliable conclusions.
We did not assess sex hormone plasma levels in order to rule out a protective effect of estrogens on LF as other authors did35. Finally, the multiplicity and efficacy of DAA combinations preclude the evaluation of the effect of specific regimens, although this shortcoming does not affect the evaluation of the fibrosis response to DAA therapy.
We conclude that in a large HCV mono and HCV/HIV coinfected cohort in which ethanol abuse was excluded, LF regression after DAA, as evaluated by NILFM, seems to be independent of gender, even from the perspective of the stage of fibrosis at baseline, the HCV features, and the HIV coinfection.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to individual privacy but are available from the corresponding author on reasonable request.
References
Hajarizadeh, B., Grebely, J. & Dore, G. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 10, 553–562. https://doi.org/10.1038/nrgastro.2013.107 (2013).
Guha, I. N. et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 47, 455–460. https://doi.org/10.1002/hep.21984 (2008).
Fontana, R. J. et al. Serum fibrosis markers are associated with liver disease progression in non-responder patients with chronic hepatitis C. Gut 59, 1401–1409. https://doi.org/10.1136/gut.2010.207423 (2010).
Larrousse, M. et al. Noninvasive diagnosis of hepatic fibrosis in HIV/HCV-coinfected patients. J. Acquir. Immune Defic. Syndr. 46, 304–311. https://doi.org/10.1097/qai.0b013e3181520502 (2007).
Castera, L., Forns, X. & Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 48, 835–847. https://doi.org/10.1016/j.jhep.2008.02.008 (2008).
Di Martino, V. et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology 40, 1426–1433. https://doi.org/10.1002/hep.20463 (2004).
Codes, L. et al. Liver fibrosis in women with chronic hepatitis C: Evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut 56, 390–395. https://doi.org/10.1136/gut.2006.101931 (2007).
Villa, E. et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 7, e44624. https://doi.org/10.1371/journal.pone.0044624 (2012).
Xiong, M. et al. Influence of gender and reproductive factors on liver fibrosis in patients with chronic hepatitis B Infection. Clin. Transl. Gastroenterol. 10, e00085. https://doi.org/10.14309/ctg.0000000000000085 (2019).
Nguyen, H. V., Mollison, L. C., Taylor, T. W., Chubb, S. A. P. & Yeap, B. B. Chronic hepatitis C infection and sex hormone levels: Effect of disease severity and recombinant interferon-alpha therapy. Intern. Med. J. 36, 362–366. https://doi.org/10.1111/j.1445-5994.2006.01093.x (2006).
Schafer, J. M. et al. Sex-biased adaptive immune regulation in cancer development and therapy. iScience. 25, 104717. https://doi.org/10.1016/j.isci.2022.104717 (2022).
Rodríguez-Torres, M. et al. Bräu N (2006) Progression to cirrhosis in Latinos with chronic hepatitis C: Differences in Puerto Ricans with and without human immunodeficiency virus coinfection and along gender. J. Clin. Gastroenterol. 40(4), 358–366. https://doi.org/10.1097/01.mcg.0000210105.66994.dc (2006).
Delarocque-Astagneau, E. et al. Past excessive alcohol consumption: A major determinant of severe liver disease among newly referred hepatitis C virus infected patients in hepatology reference centers, France, 2001. Ann. Epidemiol. 15, 551–557. https://doi.org/10.1016/j.annepidem.2004.12.006 (2005).
Poynard, T. et al. A comparison of fibrosis progression in chronic liver diseases. J. Hepatol. 38, 257–265. https://doi.org/10.1016/s0168-8278(02)00413-0 (2003).
Martín-Carbonero, L. et al. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: A European collaborative study. Clin. Infect. Dis. 38, 128–133. https://doi.org/10.1086/380130 (2004).
Andersson, K. & Chung, R. T. Hepatitis C Virus in the HIV-infected patient Clin. Liver Dis. 10, 303–20.viii. https://doi.org/10.1016/j.cld.2006.05.002 (2006).
Yen, D. W. & Sherman, K. E. Causes and outcomes of hepatic fibrosis in persons living with HIV. Curr. Opin. HIV AIDS. 17, 359–367. https://doi.org/10.1097/COH.0000000000000760 (2022).
Collazos, J., Cartón, J. A. & Asensi, V. Gender differences in liver fibrosis and hepatitis C virus-related parameters in patients coinfected with human immunodeficiency virus. Curr. HIV Res. 9, 339–345. https://doi.org/10.2174/157016211797635982 (2011).
Bachofner, J. A. et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 37, 369–376. https://doi.org/10.1111/liv.13256 (2017).
Hsu, W. F. et al. Rapid decline of noninvasive fibrosis index values in patients with hepatitis C receiving treatment with direct-acting antiviral agents. BMC Gastroenterol. 19, 63. https://doi.org/10.1186/s12876-019-0973-5 (2019).
Hsieh, Y. C. et al. Persistent liver inflammation in chronic hepatitis C patients with advanced fibrosis after direct-acting antivirals induced sustained virological response. J. Chin. Med. Assoc. 84, 472–477. https://doi.org/10.1097/JCMA.0000000000000517 (2021).
Rockey, D. C. et al. Liver biopsy. Hepatology. 49, 1017–1044. https://doi.org/10.1002/hep.22742 (2009).
Patel, K. et al. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J. Hepatol. 41, 935–942. https://doi.org/10.1016/j.jhep.2004.08.008 (2004).
Rosenberg, W. M. et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 127, 1704–1713. https://doi.org/10.1053/j.gastro.2004.08.052 (2004).
Pérez-Is, L. et al. 24-month decline of non invasive liver fibrosis markers in HCV-mono and HCV/HIV coinfection after direct-acting antiviral therapy. Sci. Rep. 12, 3828. https://doi.org/10.1038/s41598-022-07548-y (2022).
Gardner, A. R. et al. Longitudinal assessment of the enhanced liver fibrosis score in the era of contemporary HIV and hepatitis C virus treatment. J. Infect. Dis. 227, 1274–1281. https://doi.org/10.1093/infdis/jiac315 (2023).
Collazos, J. et al. Matrix metalloproteases and their tissue inhibitors in non-alcoholic liver fibrosis of human immunodeficiency virus-infected patients. World J. Virol. 6, 36–45. https://doi.org/10.5501/wjv.v6.i2.36 (2017).
Sandrin, L. et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 29, 1705–1713. https://doi.org/10.1016/j.ultrasmedbio.2003.07.001] (2003).
Castéra, L. et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128, 343–350. https://doi.org/10.1053/j.gastro.2004.11.018] (2005).
Wai, C. T. et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38, 518–526. https://doi.org/10.1053/jhep.2003.50346 (2003).
Forns, X. et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 36, 986–992. https://doi.org/10.1053/jhep.2002.36128] (2002).
Vallet-Pichard, A. et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 46, 32–36. https://doi.org/10.1002/hep.21669 (2007).
Bissell, D. M. Sex and hepatic fibrosis. Hepatology. 29, 988–989. https://doi.org/10.1002/hep.510290351 (1999).
Ashcroft, G. S. et al. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 3, 1209–1215. https://doi.org/10.1038/nm1197-1209 (1997).
Sarkar, M. et al. Sex hormone levels by presence and severity of cirrhosis in women with chronic hepatitis C virus infection. J. Viral. Hepat. 2019(26), 258–262. https://doi.org/10.1111/jvh.13027 (2019).
Lieber, C. S. Medical disorders of alcoholism. N. Engl. J. Med. 333, 1058–1065. https://doi.org/10.1056/NEJM199510193331607 (1995).
Funding
This work was supported by the Fondo de Investigaciones Sanitarias Grant (FISS-17-PI16/01999) and a research grant from VIIV Healthcare Spain, both given to Victor Asensi. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: J.C., E.V.G., V.A. Data curation: L.P.I., B.D.F., L.M., M.R.C., M.R., J.D.A, V.A. Formal analysis: J.C. Funding acquisition: V.A. Investigation: J.C., E.V.G., V.A. Resources: L.P.I., E.V.G. Software: J.C. Supervision: J.C., E.V.G., V.A. Validation: J.C., E.V.G., V.A. Visualization: V.A. Writing—original draft: L.P.I., J.C., V.A. Writing—review & editing: L.P.I.; J.C., B.D.F., L.M., M.R.C. M.R., A.R.F., G.J.F.G., S.M., J.D.A., E.V.G., V.A. Do not apply to this work in which no individual data or images are shown.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Collazos, J., Pérez-Is, L., de la Fuente, B. et al. No gender differences in the 24-month course of non-invasive liver fibrosis markers after DAA therapy in HCV-mono and HCV/HIV-coinfected patients. Sci Rep 14, 7534 (2024). https://doi.org/10.1038/s41598-024-57845-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57845-x
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.