Abstract
Radiofrequency ablation (RFA) comparative efficacy of treatments using video-assisted thoracoscopic sympathectomy (VATS) in the long term remains uncertain in patients with palmar hyperhidrosis (PHH). This study aimed to compare the efficacy and safety of RFA and VATS in patients with PHH. We recruited patients aged ≥ 14 years with diagnosed PHH from 14 centres in China. The treatment options of RFA or VATS were assigned to two cohort in patients with PHH. The primary outcome was the efficacy at 1-year. A total of 807 patients were enrolled. After propensity score matching, the rate of complete remission was lower in RFA group than VATS group (95% CI 0.21–0.57; p < 0.001). However, the rates of palmar dryness (95% CI 0.38–0.92; p = 0.020), postoperative pain (95% CI 0.13–0.33; p < 0.001), and surgery-related complications (95% CI 0.19–0.85; p = 0.020) were lower in RFA group than in VATS group, but skin temperature rise was more common in RFA group (95% CI 1.84–3.58; p < 0.001). RFA had a lower success rate than VATS for the complete remission of PHH. However, the symptom burden and cost are lower in patients undergoing RFA compared to those undergoing VATS.
Trial Registration: ChiCTR2000039576, URL: http://www.chictr.org.cn/index.aspx.
Similar content being viewed by others
Introduction
Hyperhidrosis refers to sweating exceeding physiological needs and is considered a disease of the autonomic nervous system, with an unclear specific pathogenesis1,2,3. Two previous studies reported a prevalence of 2.8% in the United States and 2.08% in China, w corresponding to 7.8 million American and 29.1 million Chinese individuals with hyperhidrosis4,5. The main symptomatic parts include the hand, axilla, craniofacial region, and feet4,5. Hyperhidrosis substantially affects patients' social life and work and may even cause depression in severe cases; severely affected patients have skin maceration and secondary microbial infections6,7.
Regarding hyperhidrosis treatment, the options are surgical and nonsurgical8,9. Nonsurgical treatment includes direct injections or through iontophoresis of botulinum toxin6,10, topical antiperspirants (aluminium chloride hexahydrate)11,12, laser treatment13, and oral medications (anticholinergics, beta-blockers, and benzodiazepines)9, and these treatments have limitations and a higher rate of recurrence. Therefore, surgical sympathectomy may be considered a final option when more conservative treatments have failed9. Video-assisted thoracoscopic sympathectomy (VATS) is the most commonly used surgical option to treat palmar hyperhidrosis (PHH)11. However, it requires general anaesthesia and has more trauma than other options14,15, and more compensatory hyperhidrosis occurs after VATS9, leading to many challenges for treatment in those patients.
As a minimally invasive procedure, radiofrequency ablation (RFA) has many satisfactory advantages, such as minor trauma and quick recovery16. The therapeutic mechanism of RFA is a thermal effect for tissue coagulation without causing neuromuscular excitation or pain17. Presently, RFA has been widely used to treat tumours and chronic pain, has achieved remarkable results18, and is effective for hyperhidrosis treatment9. However, to date, only two studies have been designed to compare the effectiveness of RFA and VATS for hyperhidrosis treatment8,19. One study reported that RFA has long-term patient satisfaction, with a success rate of 75% for treating palmar hyperhidrosis (PHH) in 46 patients8. Another nonrandomized controlled clinical trial supports the view of surgical sympathectomy as the gold-standard treatment in severe cases of PHH in 31 patients19. These studies with limited clinical data have not provided strong evidence for the management of hyperhidrosis11.
Here, we conducted a nationwide multicentre cohort study to compare the efficacy and safety of RFA and VATS in patients with PHH to improve clinical practice.
Methods
Study design
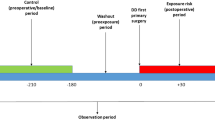
The comparative efficacy research to assess the use of RFA and VATS for PHH was an investigator-initiated, prospectively collected data, retrospective controlled cohort study done at 14 centers in China between 2015 and 2019 (Supplementary Statistical Analysis Plan).
Study participants
Eligible participants were aged 14 years or older, diagnosed with primary PHH and treated with RFA or VATS. Patients who had non-palmar hyperhidrosis and non-interventional treatment for hyperhidrosis were excluded8. The study was approved by the institutional ethics committees at the principal investigator centre on September 16, 2020, and was being recognized at 13 other centres. This study was registered with the China Clinical Trials Registry on November 1, 2020 (ChiCTR2000039576). All the patients provided written informed consent before RFA or VATS for PHH treatment, but they were not invited to be included in this study at that time. Therefore, all data were obtained after ethical approval, and patients were explained by our follow-up that they were included in this study, the ethics approve it was exempted from signing a written informed consent form. The study was performed according to the Declaration of Helsinki and Good Clinical Practice principles.
Study cohort
The treatment options were determined according to a prespecified analysis plan between the RFA and VATS frameworks (Fig. 1).
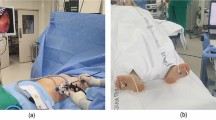
Radiofrequency ablation (RFA) and video-assisted thoracoscopic sympathectomy (VATS) for treatment, with pathways and procedures at the fourth thoracic level. R-1: Body surface for the operative approach in RFA. R-2: Lateral film for 3-dimensional X-ray computed tomography imaging in RFA. R-3: Positive film for 3-dimensional X-ray computed tomography imaging in RFA. V-1: Body surface for the operative approach in VATS (third/fourth intercostal space). V-2: Straight view of the sympathetic nerve before surgery with VATS. V-3: Straight view of the sympathetic nerve after surgery with VATS.
Patients in this study cohort included two distinct groups: the RFA group, which comprised patients who underwent RFA applied unilaterally; the control (VATS) group, which comprised patients who underwent electrosurgery sympathectomy (bilateral of Thoracic 4 and or Thoracic 3 ganglia according to the actual condition) using video-assisted thoracoscopy under general anaesthesia with double-lumen endotracheal intubation8,11. In the RFA group, RFA was performed for PHH according to local practice at the study centres because no authoritative practical guidelines were available. First, the patients whether underwent a homogeneity technique protocol at the participating centres by judge of surgeon or operator. Following subcutaneous local anaesthetic infiltration, the 5 mm active cannula of the RFA device was advanced to the bilateral of thoracic 4 sympathetic ganglions under fluoroscopic guidance (X-ray computed tomography). When the probe reached the desired point, the level of the cannula was tested by 3-dimensional image reconstruction. After neurophysiological testing8, RFA thermal coagulation was applied at 90 °C for 180 s, and 2 ml of 2% lidocaine was spread through the cannula after thermal coagulation was necessary. The decision to treat PHH was not determined by study design but instead by the physician and patient and might be influenced by regional health policy practices (Social Security fund policy and Medical Insurance Policy).
Variables
Control variables, including demographic data, clinical disease and family history, were collected preoperatively from the medical charts at each participating centre. The variables of the Hyperhidrosis Disease Severity Scale (HDSS)1 and the quality of life (QOL) questionnaire19 by follow-up to be evaluated between pre-operation and post-operation, respectively. The HDSS questionnaire comprises four statements, each receiving a score of 1–4, with 1 being the mildest grade and 4 the worst20. The QOL questionnaire comprises twenty statements, each receiving a score of 1–5, with 20 being the mildest grade and 100 the worst. The outcome variables included the clinical efficacy, safety (intraoperative and postoperative complications), patient satisfaction, HDSS and QOL questionnaire, compensatory hyperhidrosis, and symptom recurrence follow-up data at postoperative months 1, 3, 6, and 12 (1 year), as previously reported8,21,22. For patient satisfaction, the chief complaint of patients was made by phone call and return follow-up to be evaluated. To evaluate postoperative QOL, the final data of the follow-up questionnaire was used. All follow-up was conducted by an external blinded endpoint committee, established at the principal investigator's institution, to ensure unbiased assessments. For preoperative assessment, clinical data were obtained from medical records and medical documents. Postoperatively, the follow-up was carried out by investigators who had undergone standardized training, thus guaranteeing the reliability and consistency of data collection and analysis.
Outcomes measures
The primary outcome was the clinical efficacy at 1 year. Successful clinical efficacy was defined as complete remission after treatment for PHH, and ineffective treatment was defined as no remission or a few partial remissions after treatment for PHH. Surgical failure was defined as no improvement in hyperhidrosis symptoms postoperatively. The HDSS and QOL were assessed preoperatively and postoperatively as components of the primary outcome. The secondary outcomes included symptom recurrence, complications, compensatory hyperhidrosis, patient satisfaction, length of stay, and hospital costs.
Sample size calculation
The study sample included patients aged ≥ 14 years who had received RFA or VATS for PPH, assuming an efficacy rate of 68–100% with VATS according to published literature11. The power analysis for the equivalence tests of two independent proportions was performed using PASS 11 software (NCSS, LLC., Kaysville, Utah, USA), which Sample sizes of 150 in the treatment group and 150 in the control group achieved 90% power to detect equivalence. The margin of equivalence, given in terms of the difference, extended from 0.20 to 0.20. The calculations assume that two, one-sided Z tests are used. The significance level is Alpha targeted at 0.05.
Statistical analysis
The RFA and VATS cohorts were analysed separately for HDSS and QOL preoperatively and postoperatively. Patients who were lost to follow-up were excluded from the analysis. In patients with PHH, the baseline characteristics were compared between patients receiving RFA and VATS using standardized mean differences (SMDs). The propensity score for receiving RFA was estimated using a logistic regression model. Covariates included in the model were demographics (age and sex), family history, HDSS and QOL preoperatively. Propensity-score matching was implemented using a nearest-neighbour strategy, with a minimum caliper of 0.123. The ratio was one patient receiving RFA matched with one patient receiving VATS. The SMD was used to assess the balance of baseline covariates between the RFA and VATS groups in the matched cohort. An SMD of less than 0.10 indicated a good balance24. In the matched cohort, the nonnormally distributed length of stay and hospitalization costs were converted to categorical variables based on the median. The primary and secondary outcomes to compare the distributions of categorical variables using chi-squared test in the unmatched cohort and logistic regression models in the matched cohort are reported with odds ratios (ORs) and 95% confidence intervals (CIs) for the two treatment groups. To test whether the findings of the patient-level analysis might be due to a causal effect of RFA, we used a further adjusted time-varying Cox proportional hazard model to estimate the hazard ratio for ineffective treatment in the matched cohort (Statistical Analysis Plan in the Supplementary Appendix, Fig. 1).
Statistical analyses were performed using R 3.6.3 software.
Ethics approval
This study was approved by the institutional review board (IRB) of Affiliated Hospital of Guangdong Medical University (No. PJ2020-079). Data from the surgical options cohort do not involve any personally identifiable data. Thus, the IRB approved the cohort study without informed consent from participants.
Transparency statement
The manuscripts guarantors (all authors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and any discrepancies from the study as planned have been explained. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Results
Baseline characteristics
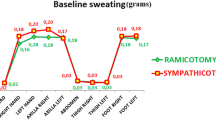
Between March 4, 2015, and December 31, 2019, at 14 centres in China, 853 patients were enrolled, and 46 patients were excluded because they were lost to follow-up. Thus, the population for our study of the clinical efficacy included 807 patients: 351 in the RFA group and 456 in the VATS group (Fig. 2, Table S1 in the Supplementary Appendix). The median follow-up was 1.5 years (IQR 1.4–1.7) in the RFA group and 1.7 (IQR 1.4–2.3) in the VATS group. 19 (5.4%) patients in the RFA group and 27 (5.9%) in the control group were lost to follow-up. Because the proportion of patients with missing items was moderate, complete case analyses were performed. In the baseline cohort, although patients in the RFA and VATS groups were well matched for disease severity (HDSS), compared with the VATS group, patients who underwent RFA were older (mean age 25.1 years (SD 6.7) versus 23.0 years (SD 5.7); SMD 34.5%), more female (187/351 [53.3%] versus 215/456 [47.2%]; SMD 12.3%), and had lower QOL scores (SMD 19.1%) (Table 1). After propensity score matching, all the baseline characteristics of patients who received RFA and VATS were well balanced, with an SMD less than 0.10 (Table 1).
Primary outcome
The postoperative association between treatment options and outcomes is presented in Table 2. In the matched cohort of 312 patients who underwent RFA and 312 patients who underwent VATS, the surgical failure rate was not significantly different between the RFA and VATS groups at the hospital (0.3% [1/312] versus 1.0% [3/312]; OR 2.84; 95% CI 0.26 to 72.59; p = 0.428). However, the rate of complete remission was 79.2% (247/312) to treat PHH in the RFA group and 91.3% (285/312) in the VATS group (OR 0.35; 95% CI 0.21 to 0.57; p < 0.001) at 1-year postoperatively. The multivariable Cox model showed that the risk of ineffective treatment was higher in the RFA group than in the VATS group (HR 2.61; 95% CI 1.61 to 4.23; p < 0.001) and higher in the group with a family history than in the group without a family history (HR 1.84; 95% CI 1.21 to 2.81; p = 0.004). However, the risk of ineffective treatment was lower with a worse quality of life (QOL > 84) than without (HR 0.41; 95% CI 0.19 to 0.91; p = 0.027) and with a skin temperature rise than without (HR 0.58; 95% CI 0.38 to 0.90; p = 0.014) (Fig. 3).
Multivariable models to estimate the hazard ratio of symptom recurrence using a time-varying Cox proportional hazard model. *1: My sweating is never noticeable and does not interfere with my daily activities; 2: My sweating is tolerable, but sometimes it interferes with my daily activities; 3: My sweating is barely tolerable and frequently interferes with my daily activities; 4: My sweating is intolerable and always interferes with my daily activities. RFA Radiofrequency ablation, VATS Video-assisted thoracoscopic sympathectomy, HDSS Hyperhidrosis Disease Severity Scale, QOL Quality of life questionnaire.
As a primary outcome component, the HDSS showed a significant reduction in the two groups preoperatively and postoperatively (p < 0.001), and quality of life (QOL questionnaire) was significantly improved in the two groups (p < 0.0001) (Table S2 in the Supplementary Appendix). However, compared with the RFA group, the postoperative HDSS (1) assessment improved more significantly in the VATS group at the end of follow-up (275/312 [88.1%] versus 245/312[78.5%]; OR 0.46; 95% CI 0.29 to 0.73; p = 0.001). Similarly, the postoperative QOL questionnaire (20–35) also improved more significantly in the VATS group (251/312[80.4%] versus 217/312[69.6%]; OR 0.54; 95% CI 0.37 to 0.78; p = 0.001) (Table 2).
Secondary outcome
In the matched cohort, the number of patients with PHH who underwent RFA was higher than that who underwent VATS regarding symptom recurrence (OR 2.78, 95% CI 1.71 to 4.63; p < 0.001). By contrast, the rates of clinical symptoms after treatment showed that the rates of palmar dryness, postoperative pain, and surgery-related complications were lower in the RFA group than in the VATS group, but the rates of skin temperature rise were higher in the RFA group (Table 2). The common complications of VATS were pneumothorax (11/312 [3.5%]), pleural effusion (6/312 [1.9%]), and incisional pain (4/312 [1.3%]) (Table S1 in the Supplementary Appendix). The length of stay was shorter in the RFA group than in the VATS group (OR 0.21; 95% CI 0.13 to 0.33; p < 0.001). The hospital costs were lower in the RFA group than in the VATS group (OR 0.01; 95% CI 0.00 to 0.01; p < 0.001). Regarding compensatory hyperhidrosis and the chief complaint of patient satisfaction, we found no significant differences between RFA and VATS (Table 2).
Discussion
Both RFA and VATS are considered successful procedures for PHH to complete remission. However, RFA (79.3%), compared with VATS (91.2%), resulted in a lower rate of complete remission for PHH 1 year postoperatively. The reason may be that the operator excises the sympathetic nerve more precisely under direct vision on the screen through video-assisted thoracoscopy25 and not just because the operator has relatively less experience eliciting this block8.
Surgery-related complications were less frequent in the two groups, a finding consistent with that in several previous studies demonstrating that the destruction of these nerves is safe and effective to stop hyperhidrosis of the palmar9,11,14. Complications have been described regarding two aspects. On the one hand, complications related to performing surgical procedures commonly occur during the perioperative period, such as dyspnoea, acute chest syndrome, incisional pain, pneumothorax, and pleural effusion. Additionally, complications can be related to excision of the sympathetic nerve, commonly appearing after hospital discharge, such as axillary pain, shoulder-back pain, compensatory hyperhidrosis, bradycardia, nasal obstruction, nerve injury, and intercostal neuralgia21. In this large sample cohort study, although not all complications were observed, they were considered tolerable because of the relative merits of improved hyperhidrosis symptoms, an improved quality of life and patient satisfaction in each patient with chief complaints.
In the present study, the advantages of RFA were its minimal invasiveness16, low postoperative painVB17, short length of stay, and low hospital cost. These advantages are reasonable and deserve positive recognition; RFA was used as a lesion generator for thermal ablation under fluoroscopic guidance using a 5 mm active cannula8. A more minimally invasive procedure that does not require general anaesthesia and greater trauma procedures14,15,26 leads to less postoperative pain17, possibly due to the unnecessary longer length of stay and reduced hospitalization costs for RFA procedures than for VATS procedures. Additionally, sympathetic vasoconstrictor reflexes after the destruction of the sympathetic ganglion in two group27, there is an increase in skin temperature in the affected areas of the patient. Interestingly, we found a higher rate of skin temperature rise in the RFA group than in the VATS group. This can be attributed to two potential factors. Firstly, the application of thermocoagulation at 90 °C for 180 s may exert a more extensive influence on the RFA of peripheral nerve tissue8. Secondly, VATS group with general anaesthesia greatly impairs thermoregulation and synchronously reduces the thresholds for vasoconstriction28. Our data showed a negative correlation between palmar temperature rise and inefficacy treatment. However, palmar temperature changes may not be used to predict a cure or guide surgical approaches29. In general, RFA options lead to a lower symptomatic burden, which is more likely to be accepted by patients, and more treatment options (one more RFA, or VATS) will be available in patients who undergo RFA when symptoms recur22.
In comparison, the advantage of VATS treatment for hyperhidrosis is that the sympathetic nerve can be excised more thoroughly, leading to more significant improvement in the symptoms of hyperhidrosis and quality of life, and the rate of symptom recurrence is lower in VATS than in RFA. A pathogenesis study of PHH showed that the cholinergic receptor nicotinic alpha 1 subunit and activin a receptor type 1 may be involved in the pathogenesis of primary hyperhidrosis30,31. Another genome-wide analysis of families with PHH showed that variants or mutations located outside the coding regions may be involved in the molecular pathogenesis of PHH3. The Cox proportional hazard model for ineffective treatment showed a higher risk in patients with a family history than in those without, likely because of variants or mutations located outside the coding regions5,30.
This study has several limitations. First, an observational study evaluating the clinical efficacy of RFA and VATS is potentially subjected to selection bias32. Although balance was achieved in each cohort by propensity score matching, patients selected for RFA may still differ regarding treatment history compared with patients receiving VATS, and seven centres did not perform RFA for PHH. However, sympathectomy is the final option of treatment when conservative treatments fail or are intolerable11, and the treatment history does not affect the clinical outcome of sympathectomy3,30. Second, the proposed different follow-up times were observed for the RFA or VATS treatment effect with HDSS and QOL must be independently assessed22. In this context, the endpoint assessment for the treatment of clinical efficacy for long-term outcomes might require increased attention9. Third, we acknowledge that the reliability of the measures should be interpreted cautiously due to the possible operator technical skills variation in this study33. Finally, missing data likely influenced the results, and we are not sure whether the inclusion of lost to follow-up data will affect the final analysis results of this study.
Conclusions
This study suggests that performing RFA had a lower success rate than VATS for the complete remission of palmar hyperhidrosis. However, the symptom burden and cost are lower in patients undergoing RFA compared to those undergoing VATS.
Data availability
Data are available through the institutional medical charts database with relevant approvals. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PHH:
-
Palmar hyperhidrosis
- RFA:
-
Radiofrequency ablation
- VATS:
-
Video-assisted thoracoscopic sympathectomy
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
- HDSS:
-
Hyperhidrosis Disease Severity Scale
- QOL:
-
Quality of life questionnaire
- SD:
-
Standard deviation
- SMD:
-
Standardized mean difference
References
Gabes, M. et al. Hyperhidrosis Quality of Life Index (HidroQoL©): Further validation and clinical application in patients with axillary hyperhidrosis using data from a phase III randomized controlled trial. Br. J. Dermatol. 184, 473–481 (2020).
Wade, R. et al. Interventional management of hyperhidrosis in secondary care: A systematic review. Br. J. Dermatol. 179(3), 599–608 (2018).
Schote, A. B. et al. Genome-wide linkage analysis of families with primary hyperhidrosis. PLoS ONE 15(12), e0244565 (2020).
Strutton, D. R., Kowalski, J. W., Glaser, D. A. & Stang, P. E. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: Results from a national survey. J. Am. Acad. Dermatol. 51(2), 241–248 (2004).
Lai, F. C. et al. Nation wide epidemiological survey of primary palmar hyperhidrosis in the People’s Republic of China. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 25(2), 105–108 (2015).
Heckmann, M., Ceballos-Baumann, A. O. & Plewig, G. Botulinum toxin A for axillary hyperhidrosis (excessive sweating). N. Engl. J. Med. 344(7), 488–493 (2001).
Walling, H. W. Primary hyperhidrosis increases the risk of cutaneous infection: A case-control study of 387 patients. J. Am. Acad. Dermatol. 61(2), 242–246 (2009).
Purtuloglu, T. et al. Effect of radiofrequency ablation and comparison with surgical sympathectomy in palmar hyperhidrosis. Eur. J. Cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 43(6), e151-154 (2013).
Hasimoto, F. N., Cataneo, D. C., Hasimoto, E. N., Ximenes, A. M. G. & Cataneo, A. J. M. Radiofrequency in the treatment of primary hyperhidrosis: Systematic review and meta-analysis. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 30(2), 111–120 (2020).
Kavanagh, G. M. & Shams, K. Botulinum toxin type A by iontophoresis for primary palmar hyperhidrosis. J. Am. Acad. Dermatol. 55(5 Suppl), S115-117 (2006).
Nawrocki, S. & Cha, J. The etiology, diagnosis, and management of hyperhidrosis: A comprehensive review: Therapeutic options. J. Am. Acad. Dermatol. 81(3), 669–680 (2019).
Gee, S. & Yamauchi, P. S. Nonsurgical management of hyperhidrosis. Thoracic Surg. Clin. 18(2), 141–155 (2008).
Cervantes, J. et al. Laser treatment of primary axillary hyperhidrosis: A review of the literature. Lasers Med. Sci. 33(3), 675–681 (2018).
Atkinson, J. L., Fode-Thomas, N. C., Fealey, R. D., Eisenach, J. H. & Goerss, S. J. Endoscopic transthoracic limited sympathotomy for palmar-plantar hyperhidrosis: Outcomes and complications during a 10-year period. Mayo Clin. Proc. 86(8), 721–729 (2011).
Cruddas, L. & Baker, D. M. Treatment of primary hyperhidrosis with oral anticholinergic medications: A systematic review. J. Eur. Acad. Dermatol. Venereol. JEADV 31(6), 952–963 (2017).
Abd-Elsayed, A., Nguyen, S. & Fiala, K. Radiofrequency ablation for treating headache. Curr. Pain Headache Rep. 23(3), 18 (2019).
Starr, J. B., Gold, L., McCormick, Z., Suri, P. & Friedly, J. Trends in lumbar radiofrequency ablation utilization from 2007 to 2016. Spine J. Off. J. N. Am. Spine Soc. 19(6), 1019–1028 (2019).
Friedman, M. et al. Radiofrequency ablation of cancer. Cardiovasc. Intervent. Radiol. 27(5), 427–434 (2004).
Garcia Franco, C. E., Perez-Cajaraville, J., Guillen-Grima, F. & España, A. Prospective study of percutaneous radiofrequency sympathicolysis in severe hyperhidrosis and facial blushing: Efficacy and safety findings. Eur. J. Cardio-thoracic Surg. Off. J. Eur. Assoc. Cardio-thoracic Surg. 40(4), e146-151 (2011).
Solish, N. et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. [et al]. 33(8), 908–923 (2007).
Puffer, R. C., Bishop, A. T., Spinner, R. J. & Shin, A. Y. Bilateral brachial plexus injury after MiraDry® procedure for axillary hyperhidrosis: A case report. World Neurosurg. 124, 370–372 (2019).
Romero, F. R., Cataneo, D. C. & Cataneo, A. J. M. Outcome of percutaneous radiofrequency thoracic sympathectomy for palmar hyperhidrosis. Semin. Thorac. Cardiovasc. Surg. 30(3), 362–366 (2018).
Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 10(2), 150–161 (2011).
Austin, P. C. & Stuart, E. A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 34(28), 3661–3679 (2015).
Horslen, L. C., Wilshire, C. L., Louie, B. E. & Vallières, E. Long-term impact of endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Ann. Thorac. Surg. 106(4), 1008–1012 (2018).
Alric, P. et al. Video-assisted thoracoscopic sympathectomy for palmar hyperhidrosis: Results in 102 cases. Ann. Vasc. Surg. 16(6), 708–713 (2002).
Baron, R. & Maier, C. Reflex sympathetic dystrophy: Skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain. 67(2–3), 317–326 (1996).
Sessler, D. I. Perioperative thermoregulation and heat balance. Lancet. 387(10038), 2655–2664 (2016).
Liu, Y. et al. Sympathicotomy for palmar hyperhidrosis: The association between intraoperative palm temperature change and the curative effect. Ann. Thorac. Cardiovasc. Surg. Off. J. Assoc. Thorac. Cardiovasc. Surg. Asia 21(4), 359–363 (2015).
Lin, J. B. et al. CHRNA1 promotes the pathogenesis of primary focal hyperhidrosis. Mol. Cell. Neurosci. 111, 103598 (2021).
Lin, J. B. et al. Involvement of activin a receptor type 1 (ACVR1) in the pathogenesis of primary focal hyperhidrosis. Biochem. Biophys. Res. Commun. 528(2), 299–304 (2020).
Agoritsas, T., Merglen, A., Shah, N. D., O’Donnell, M. & Guyatt, G. H. Adjusted analyses in studies addressing therapy and harm: Users’ guides to the medical literature. Jama 317(7), 748–759 (2017).
Stulberg, J. J. et al. Association between surgeon technical skills and patient outcomes. JAMA Surg. 155(10), 960–968 (2020).
Acknowledgements
The authors acknowledge the strict review and supervision of the Ethics Committee from the 14 participation institutions. We are grateful to Professor Liming Fan (dermatologist and senior investigator) for the constructive comments concerning this manuscript. We acknowledge the enthusiastic collaboration of all the dermatologists and specialist nurses in China who provided the data. In particular, we are grateful to all the surgical patients who were willing to participate in our study and for their enthusiasm and cooperation concerning the evaluation of the clinical outcomes during follow-up. The patients in this manuscript provided written informed consent for the publication of their case details.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82372174 and 82072208), Key Projects of Guangdong Natural Science Foundation (No. 2018B030311038), Science and Technology Plan Project of Jiaxing (No. 2020AY30011), and Science and Technology Plan Project of Zhanjiang (No. LCYJ2019B008 and 2021A05073).
Author information
Authors and Affiliations
Contributions
Professor J.T. had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: J.T., Y.Z., B.H., L.Z. Acquisition, analysis, or interpretation of the data: J.T., Y.Z., Y.Z., B.H. Drafting of the manuscript: Y.Z., J.T. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Y.Z., J.T. Administrative, technical, or material support: Y.Z., J.T. Study supervision: J.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, Y., Zhu, Y., Li, J. et al. Efficacy and safety of radiofrequency ablation versus surgical sympathectomy in palmar hyperhidrosis. Sci Rep 14, 7620 (2024). https://doi.org/10.1038/s41598-024-57834-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-57834-0
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.