Abstract
Prognostic features in advanced perihilar cholangiocarcinoma (pCCA) patients who received first-line hepatic arterial infusion chemotherapy (HAIC) are unknown. The purpose of our study was to develop an applicable score based on serum inflammatory-tumor biomarkers to predict the survival of advanced pCCA patients who received first-line HAIC. In total, 106 advanced pCCA patients were enrolled as the training cohort. The optimal cutoff values of baseline variables were defined by the receiver operating characteristic method or according to previous publications. According to the results of Cox regression analysis, baseline neutrophil-to-lymphocyte ratio (NLR) > 3.19, carcinoembryonic antigen (CEA) > 10 ng/mL, and carbohydrate antigen 19-9 (CA19-9) > 200 U/mL were identified as independent survival predictors, which were used to develop the NLCECA score (NLR, CEA, and CA19-9). When including the NLCECA score in the multivariate analysis, the NLCECA score was the only independent predictor of survival. The risk of survival decreased by 111.9% for each 1-point increase in the NLCECA score. Additionally, the NLCECA score could also predict survival in another 33 patients in the validation cohort (P < 0.001). In summary, the NLCECA score is a potential biomarker system for predicting the survival of advanced pCCA patients who received first-line HAIC.
Similar content being viewed by others

Introduction
Cholangiocarcinoma (CCA) consists of intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) CCA1. pCCA, localized to the perihilar bile duct, is the most common, accounting for approximately 50% of all CCA cases2. However, when diagnosed, most pCCA patients are at the advanced stage, and thus without the opportunity for resection, resulting in dismal survival expectations1. Currently, the first-line treatment for advanced pCCA is systemic chemotherapy using gemcitabine and cisplatin (CisGem), with a median overall survival (mOS) of less than a year3. Hepatic arterial infusion chemotherapy (HAIC), which can increase local drug concentrations, has been proven beneficial to survival in liver malignancies4,5. Wang et al.6 have demonstrated the efficacy of HAIC using oxaliplatin and 5-fluorouracil, with a median progression-free survival (mPFS) and an mOS of 12.2 and 20.5 months, respectively, in a prospective phase II trial. However, the survival benefit of HAIC for pCCA patients is variable. Hence, there is a clinical need for reliable survival biomarkers that can be used to predict the effectiveness of HAIC in pCCA patients.
In cancer patients, lymphocytes are the most responsible immune cells and can eradicate tumor cells by inhibiting cell proliferation or migration7. However, tumor-associated neutrophils can modulate the extracellular matrix and suppress the cytolytic activity of immune cells8. The neutrophil-to-lymphocyte ratio (NLR) in peripheral blood tests has been used as a convenient inflammatory biomarker because of its association with system inflammation and tumor microenvironment. Numerous reports and meta-analyses have found that an elevated NLR before treatment is related to worse OS in several malignancies9,10,11. However, whether NLR is closely related to the survival of pCCA patients who received HAIC remains unclear.
Therefore, the purpose of the study was first to evaluate the neutrophil-to-lymphocyte ratio at baseline (bNLR) as a survival biomarker and then develop an applicable score based on serum inflammatory-tumor biomarkers to predict treatment outcomes for pCCA patients receiving first-line HAIC.
Materials and methods
Study design
This retrospective study was approved by the institutional review board of Peking University Cancer Hospital (approval protocol number: 2021KT144), and the requirement for informed consent was waived by the institutional review board of Peking University Cancer Hospital. All methods were performed in accordance with the Declaration of Helsinki. The data on pCCA patients from a single center for 10 consecutive years (January 2011 to January 2020) were reviewed.
The inclusion criteria were: (1) unresectable pCCA patients, including those with Blumgart T3 Stage lesions12, N2 lymph node metastasis, intrahepatic or distant metastasis, liver cirrhosis, or decreased liver function; (2) patients with a total bilirubin level less than 100 μmol/L and albumin level greater than 30 g/L prior to HAIC treatment; (3) patients who received first-line HAIC for at least two cycles using the 3cir-OFF regimen; (4) patients who underwent peripheral blood tests within 7 days before the first cycle of HAIC procedure; and (5) patients who had abdominal contrast-enhanced computed tomography (CT) or magnetic resonance (MR) imaging within 1 month before the first cycle of HAIC and after every two cycles.
The exclusion criteria were: (1) coexistent malignancies; (2) received systemic chemotherapy, radiation therapy, resection, and other previous local treatments; (3) received HAIC other than the 3cir-OFF regimen, concomitant to other local treatment or systemic treatment; (4) absence of tumor response evaluation; and (5) a follow-up period of less than 6 months.
A total of 228 advanced pCCA patients were treated from January 2011 to January 2020, of which 31 patients only received percutaneous transhepatic cholangial drainage (PTCD), 49 patients had previously received systemic chemotherapy, radiation therapy, resection, or other local treatments, and 12 patients received HAIC with different regimens. Among the other 136 patients who received HAIC (3cir-OFF), 129 patients had abdominal imaging before the initiation of HAIC. Of the 129 patients, three had coexisting malignancies, seven did not receive a response evaluation of HAIC, and 13 patients only received one cycle of HAIC. Finally, 106 pCCA patients were enrolled (Fig. 1). According to the same inclusion and exclusion criteria, an additional 33 advanced pCCA patients who received first-line HAIC treatment at our center from January 2020 to January 2022 served as the validation cohort.
HAIC procedure
HAIC was performed by percutaneous implantation of indwelling port catheter systems as previously described13. First, all extrahepatic arteries branching out from the hepatic artery, such as the right gastric artery and accessory left gastric artery, were embolized with 0.018-inch micro-coils. Second, hepatic arterial blood flow was redistributed using 0.018-inch micro-coils to convert multiple arteries into a single arterial blood supply in cases of variation involving multiple hepatic arteries. Finally, the catheter tip was fixed in the gastroduodenal artery, and a side hole was opened in the distal part of the common hepatic artery. Subsequently, the indwelling catheter was connected to the port. The HAIC regimen was 3cir-OFF, consisting of oxaliplatin (40 mg/m2 for 2 h), 5-fluorouracil (800 mg/m2 for 22 h), and folinic acid (200 mg/m2, 2 h from the beginning of 5-fluorouracil infusion) on days 1–3 every 4 weeks14,15,16,17,18,19. A maximum of six consecutive HAIC cycles were performed on patients not showing disease progression. Maintenance therapy with oral capecitabine was followed until tumor progression.
Follow-up and assessments
Overall survival was calculated from the initiation of HAIC to death or last follow-up. PFS was defined as the period from the date of HAIC to disease progression, death, or last follow-up, whichever happened first. All patients underwent contrast-enhanced CT or MR imaging and routine laboratory studies at baseline. During the treatment phase, patients underwent regular laboratory studies, serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) level evaluation, and CT or MR imaging after every two HAIC cycles and every 3 months during capecitabine treatment. Survival follow-up was conducted every 2 months by telephone call until September 1, 2021 (January 1, 2024, for the validation cohort), or until the occurrence of death/loss to follow-up.
Statistical analysis
CEA and CA19-9 cutoff values were used similar to previous publications (CEA: 10 ng/mL; CA199: 200 U/mL)5,6. The cutoff values of other baseline variables were defined by the receiver operating characteristic (ROC) method. Univariate and multivariate analyses were performed by the Cox proportional hazards regression method. Variables with a P-value < 0.05 on univariable analysis were considered for multivariable analysis. Variables identified as independent predictors for OS were used to construct the survival scoring system. OS/PFS was assessed by Kaplan–Meier analysis. Differences with a P-value < 0.05 were considered statistically significant. All analyses were performed by SPSS v.23.0 software (IBM Corp, Armonk, NY, USA).
Ethics declarations
This retrospective study was approved by the institutional review board of Peking University Cancer Hospital (approval protocol number: 2021KT144).
Consent to participate
This retrospective study was approved by the institutional review board, and the requirement for informed consent was waived.
Results
Patients
All 106 pCCA patients received HAIC (3cir-OFF) as first-line treatment. The final follow-up was completed on September 1, 2021. The median follow-up time was 59.3 months. Until last follow-up, 82 (77.4%) patients died, and 80 (75.5%) patients had progressed. The patients received a median of five cycles (range, two to six cycles) of HAIC. Of the 106 patients (mean age, 60.0 ± 10.4 years), 69 (65.1%) were male and 37 (34.9%) were female. The number of patients with Child–Pugh class A and B was 37 (34.9%) and 69 (65.1%), respectively. Forty-four (41.5%) patients had hepatitis B virus (HBV). The number of patients with albumin-bilirubin (ALBI) grades 1, 2, and 3 was 24 (22.6%), 77 (72.6%), and 5 (4.7%), respectively. There were 56 (52.8%) patients in locally advanced stage, 19 (17.9%) had N1 lymph node metastasis, and 31 (29.2%) had N2 lymph node metastasis or distant metastasis. The cutoff value of bNLR was defined as 3.19 by the receiver operating characteristic (ROC) method (area under the curve, 0.638; sensitivity, 0.688; specificity, 0.655.) according to 1-year survival after HAIC. Patients in the whole cohort were divided into a low bNLR group (bNLR ≤ 3.19; n = 53) and high bNLR group (bNLR > 3.19; n = 53) for further analysis (Table 1). The characteristics of patients in the validation cohort are presented in Table S1.
Relationship between bNLR, disease extent, and survival
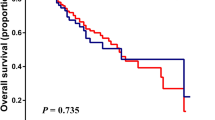
For these 106 pCCA patients, the median OS was 16.5 months [95% confidence interval (CI) 12.6–20.4] (Fig. 2A), and the median PFS was 10.2 months [95% CI 7.9–12.5] (Fig. 2B). The OS of patients in the low bNLR group (53 patients) was longer than that of the high bNLR group (53 patients) [23.7 months (95% CI 17.5–29.9 months) vs. 12.7 months (95% CI 11.2–14.2 months), hazard ratio (HR) 1.801, P = 0.007] (Fig. 2C). The PFS of the low bNLR group was also longer than that of the high bNLR group [15.0 months (95% CI 10.4–19.6 months) vs. 6.2 months (95% CI 2.5–9.9 months), HR 2.099, P = 0.001] (Fig. 2D). In addition, the mOS of patients with a locally advanced stage, N1 lymph node metastasis, and N2 lymph node metastasis or distant metastasis was 20.5 months (95% CI 16.0–25.0 months), 14.8 months (95% CI 10.5–19.1 months), and 12.3 months (95% CI 9.9–14.7 months), respectively (P = 0.029; Fig. 3A). The mPFS of patients with a locally advanced stage, N1 lymph node metastasis, and N2 lymph node metastasis or distant metastasis was 18.7 months (95% CI 10.3–27.1 months), 9.5 months (95% CI 6.3–12.7 months), and 6.9 months (95% CI 7.9–12.5 months), respectively (P < 0.001; Fig. 3B).
(A,B) Cumulative survival curves of hepatic arterial infusion chemotherapy for perihilar cholangiocarcinoma. (C,D) Cumulative survival curves of hepatic arterial infusion chemotherapy for perihilar cholangiocarcinoma low neutrophil-to-lymphocyte ratio at baseline and high neutrophil-to-lymphocyte ratio at baseline groups. bNLR neutrophil-to-lymphocyte ratio at baseline, CI confidence interval, PFS progression-free survival.
Development of the NLCECA score (NLR, CEA, and CA19-9)
The extent of disease (HR 1.387, 95% CI 1.080–1.782, P = 0.010), macroscopic growth patterns (HR 1.895, 95% CI 1.219–2.948, P = 0.005), CEA > 10 ng/mL (HR 2.285, 95% CI 1.376–3.796, P = 0.001), CA19-9 > 200 U/mL (HR 2.457, 95% CI 1.475–4.093, P = 0.001), and NLR > 3.19 (HR 1.801, 95% CI 1.164–2.786, P = 0.007) were identified as risk factors for worse OS via univariate analysis. In the multivariate analysis, CEA > 10 ng/mL (HR 1.961, 95% CI 1.135–3.386, P = 0.016), CA19-9 > 200 U/mL (HR 2.264, 95% CI 1.320–3.883, P = 0.003), and NLR > 3.19 (HR 1.729, 95% CI 1.047–2.854, P = 0.032) were identified as independent predictors of worse OS (Table 2).
Given that CEA, CA19-9, and bNLR were independent predictors for OS, a simple scoring method was developed to predict the survival of pCCA patients. Because the three factors had approximately similar hazard ratios (1.729, 1.961, and 2.264) in multivariable analysis of OS, one point was assigned to patients with NLR > 3.19, CEA > 10 ng/mL, and CA-199 > 200 U/mL. Thus, a patient could achieve either 0 (N = 18), 1 (N = 35), 2 (N = 41), or 3 points (N = 8). This simple score was named the NLCECA score (Table 3).
Relationship between the NLCECA score and survival
The mOS of patients with 0, 1, 2, 3 points were 40.8 months (95% CI 25.3–56.3 months), 22.3 months (95% CI 16.0–28.6 months), 11.5 months (95% CI 9.5–13.5 months), and 5.2 months (95% CI 0–14.8 months), respectively (P < 0.001; Fig. 4A). The mPFS of patients with 0, 1, 2, 3 points were 20.5 months (95% CI 6.5–34.5 months), 14.0 months (95% CI 9.3–18.7 months), 6.5 months (95% CI 1.2–11.8 months, and 5.1 months (95% CI 3.4–6.8 months), respectively (P < 0.001; Fig. 4B). When including the NLCECA score in the multivariate analysis, the NLCECA score was the sole independent predictor of survival, and the risk of survival decreased by 111.9% for each 1-point increase in the NLCECA score (Table 4).
(A,B) Cumulative survival curves of hepatic arterial infusion chemotherapy for 106 perihilar cholangiocarcinoma patients at different time points. (C,D) Cumulative survival curves of hepatic arterial infusion chemotherapy for perihilar cholangiocarcinoma in the validation cohort at different time points.
The usefulness of the NLCECA score was further validated in another 33 advanced pCCA patients who received first-line HAIC. The thirty-three patients in the validation cohort consisted of nine patients with 0 points, 11 patients with 1 point, seven patients with 2 points, and six patients with 3 points. For these 33 pCCA patients in the validation cohort, the median OS was 15.8 months (95% CI 12.7–18.9 months), and the median PFS was 11.5 months (95% CI 8.5–14.5 months). A higher NLCECA score in the validation cohort also indicated dismal survival. The mOS of patients with 0, 1, 2, and 3 points was 22.7 months (95% CI 22.1–23.3 months), 15.8 months (95% CI 11.5–20.1 months), 9.6 months (95% CI 5.0–14.2 months), and 7.5 months (95% CI 5.2–9.8 months), respectively (P < 0.001; Fig. 4C). The mPFS of patients with 0, 1, 2, and 3 points was 16.5 months (95% CI 12.2–20.8 months), 11.6 months (95% CI 11.3–11.9 months), 5.8 months (95% CI 4.0–7.6 months), and 5.5 months (95% CI 1.3–9.7 months), respectively (P < 0.001; Fig. 4D).
Treatment toxicity
As shown in Table S2, the most common adverse event was nausea, which occurred in 65 (61.3%) patients. No treatment-related deaths occurred during first-line HAIC treatment. Additionally, 31 patients (29.2%) incurred Grade 3 or 4 adverse events. Elevated ALT/AST were the most common Grade 3 or 4 adverse events, occurring in 16 (15.1%) patients.
Discussion
This retrospective study demonstrated NLR as a biomarker of survival for advanced pCCA patients receiving HAIC. Based on NLR, CEA, and CA19-9, which were identified as independent predictors of survival of pCCA after HAIC treatment, a simple and easily applicable clinical serum inflammatory-tumor biomarker system, the NLCECA score, consisting of NLR, CEA, and CA19-9, was established. When it was incorporated, the NLCECA score was the sole independent predictor of survival, and the risk of survival decreased by 111.9% for each 1-point increase in NLCECA score.
It is usually difficult to perform a core needle biopsy to obtain pCCA tissues due to its particular perihilar anatomical site and its periductal infiltration growth pattern along the bile duct wall. Most pathological diagnoses of advanced pCCA are based on cytopathology. Hence, for patients with advanced unresectable pCCA, there is a need for biomarkers of survival features. CEA and CA19-9 are widely used traditional prognostic biomarkers for pCCA. However, for the clinical application of CEA or CA19-9 alone, only 30% of CCA patients exhibit elevated CEA levels20 and CA19-9 is often falsely elevated in benign biliary disease or cholangitis21. Thus, a single indicator of CEA or CA19-9 may not well reflect the prognosis of pCCA patients.
Inflammation, which is one of the seven essential characteristics of tumors, can contribute to cancer progression22. CCA is also associated with biliary tract inflammation. Most cases of CCA arise during chronic infection of the biliary tree such as cholelithiasis, infection with liver fluke Clonorchis sinensis, or primary sclerosing cholangitis23. Various inflammatory biomarkers such as NLR, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio could effectively reflect the degree of inflammation and immune response and are recommended as predictive biomarkers for cancer patients24,25. Of the various inflammatory biomarkers, NLR has drawn a large amount of attention because of the function and characteristics of neutrophils and lymphocytes in tumor development. Previous studies have revealed that high neutrophil counts are associated with immunosuppression conditions26. Lymphocytes recognize and eliminate tumor cells, as well as inhibit cancer cell proliferation by producing cytokines27. Therefore, low lymphocyte counts may be related to impaired adaptive immunity activation28. Thus, high NLR levels serve as a potential biomarker of worse prognosis, which is similar to the results of our study.
The prognosis of advanced pCCA is associated with the proliferation and metabolism of the tumor, as well as the local and systemic inflammatory immune status29. CEA and CA19-9 are the main protein products of tumor metabolism secreted into the peripheral blood during tumor proliferation and progression30,31 and have been used as prognosis-related biomarkers in biliary tract cancer32. NLR in peripheral blood is associated with tumor immune microenvironment and system inflammation, which has been used as a convenient inflammatory biomarker9,10,11. In our study, a new serum inflammatory-tumor biomarker system, the NLCECA score based on NLR, CEA, and CA19-9, was established, and it accurately reflected the tumor growth and proliferation level, as well as the body and tumor inflammatory immune status. When it was incorporated, the NLCECA score was the sole independent predictor of survival, with the risk of survival decreasing by 111.9% for each 1-point increase in the NLCECA score. As a liquid marker, it could aid in the selection of advanced pCCA patients for first-line HAIC in clinical practice. Moreover, due to the easily accessible clinical laboratory test indexes adopted in this score, it is convenient for clinical application.
Our research has several limitations. First, because this was single-center retrospective research, results require further validation in more extensive, multi-center studies. Second, the association of neutrophils to each subgroup lymphocyte ratio with the survival of pCCA patients was not further explored in this study.
In summary, as an immune and inflammatory indicator, low bNLR is an independent predictor of better survival of pCCA after HAIC treatment. A new established serum inflammatory-tumor biomarker system, the NLCECA score, consisting of NLR, CEA, and CA199, may be a reliable survival prediction system for advanced pCCA after HAIC treatment. Prospective validation of the NLCECA score is also warranted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rizvi, S., Khan, S. A., Hallemeier, C. L., Kelley, R. K. & Gores, G. J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 15, 95–111 (2018).
Banales, J. M. et al. Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 13, 261–280 (2016).
Palmieri, L. J. et al. The choice for the optimal therapy in advanced biliary tract cancers: Chemotherapy, targeted therapies or immunotherapy. Pharmacol. Ther. 210, 107517 (2020).
Hu, J. G. et al. Hepatic arterial infusion chemotherapy using oxaliplatin plus 5-fluorouracil versus transarterial chemoembolization/embolization for the treatment of advanced hepatocellular carcinoma with major portal vein tumor thrombosis. Cardiovasc. Interv. Radiol. 43, 996–1005 (2020).
Zheng, K. L. et al. Hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced gallbladder cancer. Cardiovasc. Interv. Radiol. 44, 271–280 (2020).
Wang, X. D. et al. Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology 283, 580–589 (2017).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Powell, D. R. & Huttenlocher, A. Neutrophils in the tumor microenvironment. Trends Immunol. 37, 41–52 (2016).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 106, dju124 (2014).
McNamara, M. G. et al. Neutrophil/lymphocyte ratio as a prognostic factor in biliary tract cancer. Eur. J. Cancer 50, 1581–1589 (2014).
Tan, D. W. et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: A meta-analysis. Sci. Rep. 6, 33789 (2016).
Jarnagin, W. R. et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 234, 507–517 (2001) (discussion 517–519).
Hu, J. G. et al. Evaluation of percutaneous unilateral trans-femoral implantation of side-hole port-catheter system with coil only fixed-catheter-tip for hepatic arterial infusion chemotherapy. Cancer Imaging 19, 15 (2019).
Qin, B. L. et al. In-vitro schedule-dependent interaction between oxaliplatin and 5-fluorouracil in human gastric cancer cell lines. Anti-Cancer Drugs 17, 445–453 (2006).
Tsimberidou, A. M. et al. A phase 1 study of hepatic arterial infusion of oxaliplatin in combination with systemic 5-fluorouracil, leucovorin, and bevacizumab in patients with advanced solid tumors metastatic to the liver. Cancer 116, 4086–4094 (2010).
Kerr, D. J. et al. Phase I clinical and pharmacokinetic study of leucovorin and infusional hepatic arterial fluorouracil. J. Clin. Oncol. 13, 2968–2972 (1995).
Kingham, T. P., D’Angelica, M. & Kemeny, N. E. Role of intra-arterial hepatic chemotherapy in the treatment of colorectal cancer metastases. J. Surg. Oncol. 102, 988–995 (2010).
Carnaghi, C. et al. The efficacy of hybrid chemotherapy with intravenous oxaliplatin and folinic acid and intra-hepatic infusion of 5-fluorouracil in patients with colorectal liver metastases: A phase II study. Investig. New Drugs 25, 479–485 (2007).
Del Freo, A. et al. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo 20, 743–746 (2006).
Blechacz, B. & Gores, G. J. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 48, 308–321 (2008).
Malaguarnera, G. et al. Serum markers of intrahepatic cholangiocarcinoma. Dis. Markers 34, 219–228 (2013).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Clements, O., Eliahoo, J., Kim, J. U., Taylor-Robinson, S. D. & Khan, S. A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 72, 95–103 (2020).
Kishton, R. J., Sukumar, M. & Restifo, N. P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26, 94–109 (2017).
Wagner, D. D. New links between inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 25, 1321–1324 (2005).
Gross, R. L. & Newberne, P. M. Role of nutrition in immunologic function. Physiol. Rev. 60, 188–302 (1980).
Santoni, M. et al. Pre-treatment neutrophil-to-lymphocyte ratio may be associated with the outcome in patients treated with everolimus for metastatic renal cell carcinoma. Br. J. Cancer 109, 1755–1759 (2013).
Sürücü, E., Demir, Y. & Şengöz, T. The correlation between the metabolic tumor volume and hematological parameters in patients with esophageal cancer. Ann. Nucl. Med. 29, 906–910 (2015).
Saengboonmee, C., Sawanyawisuth, K., Chamgramol, Y. & Wongkham, S. Prognostic biomarkers for cholangiocarcinoma and their clinical implications. Expert Rev. Anticancer Ther. 18, 579–592 (2018).
Luo, G. P. et al. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim. Biophys. Acta Rev. Cancer 1875, 188409 (2021).
Moretto, R. et al. CEA increase as a marker of disease progression after first-line induction therapy in metastatic colorectal cancer patients. A pooled analysis of TRIBE and TRIBE2 studies. Br. J. Cancer 125, 839–845 (2021).
Thol, F. et al. Outcomes in patients receiving palliative chemotherapy for advanced biliary tract cancer. JHEP Rep. 4, 100417 (2021).
Acknowledgements
This study has received funding from the National Natural Science Foundation of China (No. 82172039), the Beijing Hospital Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202117), and the Beijing Natural Science Foundation (No. 7212198). We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
S.F. and J.L. conceptualized the study. H.F., B.L., G.C., L.X., Y.Z., and C.N. collected and analyzed data. S.F. and K.Z. drafted the manuscript, which was critically revised by X.W. All authors read and agreed to submit the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, S., Li, J., Fan, H. et al. NLCECA score: a serum inflammatory-tumor biomarker score to predict survival of advanced perihilar cholangiocarcinoma after hepatic arterial infusion chemotherapy. Sci Rep 14, 4466 (2024). https://doi.org/10.1038/s41598-024-53883-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-53883-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.