Abstract
The market for plant-based beverages (PBBs) is relatively new; hence, to enable its further development, it is important to use new raw materials and improve production technology. The use of natural biotechnological processes can diversify the segment of PBBs, which may offer products with better functionality than those available in the market. Therefore, the present study aimed to determine the effects of fermentation and germination on the nutritional properties of bean-based beverages (BBs) and lentil-based beverages (LBs). The applied processes significantly (p ≤ 0.05) influenced the characteristics of PBBs. Fermentation improved the antioxidant properties (e.g., by increasing the level of 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity by 2–6% and 3–7% for BBs and LBs, respectively) and modified the fatty acid (FA) profile of PBBs. This process increased the share of polyunsaturated FAs in the sn2 position in triacylglycerols, which may promote its absorption in the intestine. The simultaneous use of germination and fermentation was most effective in decreasing oligosaccharide content (< 1.55 mg/kg), which may reduce digestive discomfort after consuming PBBs. We recommend that the designing of innovative legume-based beverages should include the application of fermentation and germination to obtain products with probiotic bacteria and improved nutritional properties.
Similar content being viewed by others
Introduction
Natural biotechnological processes have been used for centuries to increase the nutritional value of foods and extend their shelf life. It is hypothesized that as farming started to replace hunting and gathering around 10,000 years ago, humans began to produce fermented foods and beverages1. Sprouting (also known as germination) of seeds has been known for a very long time, mainly in Eastern countries where germinated plants have been used in traditional cuisine2. Presently, these techniques are being successfully used to process plant and animal products3,4; thus, it may be beneficial to use these techniques to produce novel healthy foods.
Plant-based diets are becoming increasingly popular in the global food market; consequently, plant-based substitutes for animal products are in great demand5. The most popular products in this food group are milk substitutes, which are also known as plant-based beverages (PBBs). PBBs are water-soluble extracts of shredded plant materials (e.g., legumes, seeds, cereals, pseudocereals, and nuts) in the form of colloidal suspensions or emulsions6,7. Among the nondairy matrices, legumes are the least explored ones, with the majority of studies focused on soybean alone5,8. However, the market share of soy beverages is decreasing because of health concerns related to genetically modified organisms, allergens, high levels of isoflavones, and CO2 footprint9. Thus, there is an opportunity to expand and diversify this segment by using other legumes that may provide better functionality and nutrition than soy. Hence, it is necessary to gain more knowledge regarding the processing, functionality, and health benefits and risks of beverages prepared using other legumes10.
Currently, legume consumption is minimal because of limitations such as taste, availability, flatulence factor, and lack of knowledge regarding the preparation process. Designing new legume-based beverages is a beneficial approach that can overcome these limitations by offering modern convenience food10. Various methods have been used to improve the properties of legumes, such as soaking, cooking, fermentation, and germination11. The use of these processes, particularly in combination, can provide improved nutritional value, aroma, taste, texture, stability, and safety against microbial contamination7,12.
The market for PBBs is relatively new; therefore, to enable its further growth and development, it is essential to improve the technology of producing PBBs. A goal of the food industry is to develop new, ecofriendly processing strategies to reduce energy consumption and maximize the value of raw materials13. The answer to this may be the use of natural biotechnological processes such as germination and fermentation, which can increase the attractiveness of legume-based beverages and does not require the use of technologies with limited industrial application due to high energy consumption and cost related to complex equipment purchase13,14.
Previous research on the properties of legume-based beverages other than those made from soybean does not provide clear recommendations on using processes to improve product properties and overcoming barriers to their consumption. Moreover, to date, the effects of a combination of different biotechnological processes on the properties of bean-based beverages (BBs) and lentil-based beverages (LBs) have not been studied. The study focused on a BBs and LBs due to their potential to produce highly nutritious PBBs from legumes other than soy. This is the result of the high content of protein, carbohydrates, dietary fiber, and bioactive ingredients (such as polyphenols) in beans and lentils10,11. We believe that these legumes hold promise in addressing the growing demand for sustainable and nutritious food alternatives, due to their nutritional richness, diversity, cultural significance, availability, and the potential for further advancement in the development of protein-enriched plant-based foods. Therefore, the present study aimed to assess the possibility of using natural biotechnological processes, i.e., fermentation and germination, to improve the nutritional properties of BBs and LBs. The influence of the studied processes on fermentation (pH and population of lactobacilli and bifidobacteria), nutritional profile (carbohydrate content, total share of fatty acids, fatty acid profile, and positional distribution of fatty acids in triacylglycerols (TAGs)), and antioxidant properties (total phenolic content and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity (RSA)) of BB and LB was analyzed. The changes in the tested properties of the PBBs after their production and after 21 days of refrigerated storage at 6 °C were analyzed. This study also aimed to offer recommendations for the use of fermentation and germination for producing innovative legume-based beverages.
Materials and methods
Materials and experimental design
The PBBs used in our study were prepared from white kidney beans “Piękny Jaś Karłowy” (Lestello Sp. z o.o., Cmolas, Poland) and brown lentils (Natural Expert, Bialystok, Poland). The nutritional values of the tested beans and lentils are shown in Supplementary Table S1 online, according to the product label.
Three industrial freeze-dried starter cultures were used: Beaugel Soja 1 (Ets Coquard, Villefranche-sur-Saône, France), which comprised Lactobacillus casei (currently classified as Lacticaseibacillus casei), Streptococcus thermophilus, and Lactobacillus delbrueckii subsp. bulgaricus; YO-MIX 207 LYO 500 DCU (DuPont™ Danisco, Copenhagen, Denmark), which comprised S. thermophilus, L. delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, and Bifidobacterium lactis; and ABY-3 (Chr. Hansen, Hørsholm, Denmark), which comprised L. acidophilus La-5, Bifidobacterium animalis subsp. lactis BB-12, S. thermophilus, and L. delbrueckii subsp. bulgaricus. These starter cultures were selected to determine the differences between fermentation with a starter culture containing basic yoghurt bacteria (Beaugel Soja 1) and fermentation with starter cultures enriched with different strains of bifidobacteria (YO-MIX 207 and ABY-3).
Two variants of each PBB were prepared: from germinated (G) and non-G beans and lentils. The experimental design used in the study was based on three factors: germination, starter culture used for fermentation, and storage period at 6 °C (Table 1). The experiment was conducted in duplicate. Supplementary Table S2 online shows the physicochemical characteristics of basic PBBs derived from G beans (BBG), non-G beans (BB), G lentils (LBG), and non-G lentils (LB).
Preparation of PBBs
Germination was performed in a sprouter at 25 °C for 72 h (water was changed every 24 h). G and non-G beans and lentils were sterilized at 121 °C for 15 min, mixed with drinking water at the ratio of 1:9 (m/m), and blended until a homogeneous mass was obtained. The resulting mass was filtered through a sieve with 0.1 mm mesh size and then homogenized using the laboratory mixer L4R (Silverson, Chesham, UK). The prepared PBBs were then sterilized at 121 °C for 15 min.
Fermentation of PBBs
Inoculums were prepared by dissolving the freeze-dried starter cultures in a sterile saline solution. The PBBs were inoculated with 1.0% (m/m) of starter cultures (final cell density of around 6–7 log CFU/mL) and incubated at 45 °C for 6 h. After fermentation, the PBBs were refrigerated at 6 °C and stored for 21 days. The non-stored PBBs were frozen at − 18 °C until analysis.
Active acidity and microflora population analysis
The active acidity was determined by measuring the pH of the PBBs using a calibrated digital CPO-505 pH meter (Elmetron, Zabrze, Poland). The measurement was conducted twice.
Microflora population was analyzed by the culture method in Petri dishes. De Man, Rogosa, and Sharpe (MRS) agar (Merck, Darmstadt, Germany) was used to determine the viable population of lactobacilli. M17 agar (Merck) was used to determine the viable population of S. thermophilus. Bifidus Selective Medium (BSM) agar (Merck) was used to determine the viable population of bifidobacteria. The measurement was conducted twice. Petri dishes were incubated at 37 °C for 72 h under aerobic conditions for M17 medium and under anaerobic conditions for MRS and BSM media. After incubation, the number of colonies was counted, and CFU/mL was calculated. The result was expressed as a logarithm of the total cell count.
Carbohydrate content of PBBs
The carbohydrate content was determined by high-performance liquid chromatography (HPLC) coupled with a refractive index detector (RID). The sample was prepared according to the method of Ziarno et al.15 without any modifications. The analytes were separated using an HPLC kit equipped with DeltaChrom™ pumps, an S 6020 needle injection valve dosing loop (Sykam, Fürstenfeldbruck, Germany), a DeltaChrom™ temperature control unit column temperature controller (Sykam), and a 05397–51 Cosmosil Sugar-D column (250 × 4.6 mm, 5 µm; Cosmosil, Nacalai Tesque, Kyoto, Japan) secured by a pre-column 05394–81 Cosmosil Guard Column Sugar-D (10 × 4.6 mm, 5 µm, Cosmosil). The analytes were detected with an S3580 RID (Sykam). The chromatographic analysis parameters were the same as those described by Ziarno et al.15 without any modifications. The analysis was performed in duplicate. After the analysis, the carbohydrates were identified by comparison with the retention times of selected carbohydrate standards, including glucose, sucrose, raffinose, stachyose, and verbascose (Sigma-Aldrich, Burlington, VT, USA).
Analysis of antioxidant properties
The antioxidant properties of the PBBs were analyzed by determining total phenolic content (TPC) and the level of DPPH RSA according to the method of Zhao and Shah16 with some modifications. The PBB extracts were prepared by mixing 2 mL of the sample with 4 mL of 80% methanol; the extract was then vortexed for 1 min and centrifuged in an MPW-350R centrifuge (MPW Med. Instruments) at 4000 × g for 20 min at 4 °C. The precipitate was then extracted twice with 2 mL of 80% methanol, and the supernatants were pooled. All supernatants were filtered through a syringe filter (Merck) with 0.45 µm pore size and were used as the antioxidant extracts (AEs) to estimate TPC and DPPH RSA.
TPC (analyzed by the Folin–Ciocalteu method) and the efficiency of the PBBs to scavenge DPPH radicals were determined according to the method of Zhao and Shah16 without any modifications. The analyses were performed in duplicate. TPC was estimated by comparing the absorbance of the AEs with a calibration curve constructed using a gallic acid standard (0.5–2.5 mg/mL) and was expressed as milligrams of gallic acid (GAE)/mL of PBBs. RSA was expressed as percent DPPH inhibition using the following Eq. (1):
where A0 is the absorbance of a methanolic solution of DPPH radicals without sample extract and As is the absorbance of PBBs.
Analysis of fatty acid (FAs) profile
The PBBs were first extracted using the Folch method17. The FAs profile was analyzed according to the method of Ziarno et al.18 by using a gas chromatography system (YL6100 GC) with an installed flame ionization detector (Young Lin Instrument Co., Ltd., Anyang, Korea) and a MEGA-10 capillary column (ID 0.25 mm, film thickness 0.25 μm, length 60 m, MEGA S.r.l., Legnano, Italy). FAs were detected based on the retention time by comparison with a selected external standard (Fatty Acid Methyl Esters Standard Mixture, Merck). The analysis was performed in duplicate. The share of the analyzed FA in the FA profile was estimated by determining the area of the peaks in the chromatogram. The analysis was performed for basic PBBs obtained from G and non-G beans and lentils and for beverages fermented with the ABY-3 starter culture, which showed the highest acidification activity.
Analysis of the positional distribution of FAs in TAGs
The hydrolysis of TAGs and the analysis of the positional distribution of FAs in TAGs were conducted and calculated according to the method of Ziarno et al.18 without any modifications. The analysis was performed for basic PBBs obtained from G and non-G beans and lentils and for beverages fermented with the ABY-3 starter culture (BB102/LB102, BB102s/LB102s, BBG102/LBG102, and BBG102s/LBG102s), which showed the highest acidification activity.
Statistical analysis
Statistica version 13.1 software (StatSoft, Krakow, Poland) was used to analyze the data obtained in the experiments. Analysis of variance (ANOVA) was performed to determine the effect of germination (GM), fermentation (C), and storage (S) on the obtained results. Differences were considered significant at α = 0.05 based on Tukey’s test.
Results and discussion
Active acidity and viable bacterial population
Legume-based beverages provide a favorable matrix for fermentation; however, the process typically requires a longer time (approximately 12–48 h) for completion as compared to milk fermentation (approximately 4–6 h)6,19. During the fermentation of food (including milk and milk substitutes), it is beneficial to maintain pH in the range of 4.3–4.7; an acidic pH can protect the product from microbial contamination and retain favorable organoleptic properties related to the concentration of organic acids and other flavor-forming components20,21.
Table 2 shows the pH values and the population of lactic acid bacteria (LAB) and bifidobacteria in the tested PBBs. All fermented samples of PBBs showed a significant decrease in pH, which indicated the metabolic activity of microorganisms in all tested starter cultures. In all tested PBBs, no significant changes in pH were observed after storage. Fermentation was the primary factor that affected the obtained values (η2 ≈ 0.925–0.989).
At the time of consumption, probiotic products must contain an adequate number of viable cells, ranging from at least 106 to 107 CFU/mL, to exert their beneficial effects21. Here, by using the starter cultures YO-MIX 207 (BB/LB/BBG/LBG101) and ABY-3 (BB/ LB/BBG/LBG102), the recommended number of viable cells of the genera Lactobacillus, Streptococcus, and Bifidobacterium was achieved after fermentation of all tested samples (BBs/LBs and BBGs/LBGs) (Table 2). Most of these samples did not show a decrease in the bacterial population below 107 CFU/mL during the storage period. The BB100, BBG100, and LB100 samples fermented with the Beaugel Soja 1 starter culture did not reach the recommended Lactobacillus cell count and showed values of 5.99, 5.86, and 5.73 log10 CFU/mL, respectively. All the tested samples of PBBs showed no significant effect of germination on the population of LAB and bifidobacteria. The 21-day refrigerated storage period also had no significant effect on the pH of all tested samples; however, it showed a slight effect (η2 ≈ 0.211–0.358) on changes in the viable population of bacteria.
In the present study, the required population level of LAB and bifidobacteria and the pH value recommended for fermented beverages were achieved for most of the tested samples. Moreover, these values were obtained by fermentation performed for 6 h. Most of the PBBs described in the literature exhibited a lower acidification rate, slow growth of probiotic bacteria, and prolonged fermentation time due to the low concentration of soluble carbohydrates12,21. A short fermentation time is more efficient and economically advantageous for use on a production scale.
G and non-G BBs and LBs fermented with the Beaugel Soja 1 starter culture showed a lower efficiency in obtaining the recommended pH and level of the bacterial population. This starter culture was the least diverse one with regard to microbial species and contained only Lactobacillus and Streptococcus. The other tested starter cultures (YO-MIX 207 and ABY-3) also contained the genus Bifidobacterium; this finding indicates that a greater diversity of microflora in the starter culture is conducive to a more effective fermentation of PBBs. This phenomenon may be due to the synergistic activities of microorganisms in complex starter cultures and the metabolism of carbohydrates in legumes. According to Adamberg et al.22, the strain balance and activities of microorganisms are determined by an interplay of different factors between consortium members, such as antagonism, competition for substrates, and symbiosis by cross-feeding. In their study, the authors showed that the growth of L. paracasei F8 was enhanced in the presence of B. breve 46. Similarly, in our study, a reduced concentration of Lactobacillus cells was observed only in samples fermented using the Beaugel Soja 1 starter culture, which did not contain bifidobacteria.
The samples fermented with Beaugel Soja 1 also had the slowest rate of pH decrease. According to Pokusaev et al.23, bifidobacteria metabolize carbohydrates more efficiently than LAB. The bifidobacterial pathway yields 2.5 mol of ATP, 1.5 mol of acetate, and 1 mol of lactate from 1 mol of fermented glucose. Homofermentative LAB produce 2 mol of ATP and 2 mol of lactic acid from 1 mol of glucose, while heterofermentative LAB produce 1 mol each of lactic acid, ethanol, and ATP from 1 mol of fermented glucose. Efficient milk fermentation can be achieved using the classic yogurt culture, which is characterized by protosymbiosis between Streptococcus thermophilus and L. delbrueckii subsp. bulgaricus24. The present study shows that for the fermentation of plant-based beverages, it is more effective to use starter cultures enriched with bifidobacteria, which can enhance fermentation rate and microbial proliferation.
Carbohydrate content
Carbohydrates are the main component of legumes (55–65%) and consist mainly of starch, monosaccharides, disaccharides, and α-galactosides25. Humans and monogastric animals lack α-galactosidase required to hydrolyze α(1 → 6)glycosidic linkages; consequently, oligosaccharides remain undigested in their upper gastrointestinal tract. The undigested oligosaccharides are responsible for digestive discomfort26; therefore, it is desirable to remove these oligosaccharides during the production of legume-based products.
Table 3 shows the content of carbohydrates in the tested PBBs. BBs and BBGs showed the highest glucose content among all the carbohydrates tested. In BBs, germination was the primary factor that influenced the changes in the concentrations of glucose, sucrose, stachyose, and verbascose (η2 > 0.825). The germination process significantly reduced the content of glucose, sucrose, and verbascose and increased the content of stachyose. Fermentation with different starter cultures showed the greatest effect on reducing glucose and sucrose content in most of the tested BB and BBG samples; this decrease was directly caused by the metabolic activity of microorganisms present in the starter cultures used for fermentation.
LBs and LBGs also exhibited the highest glucose content among all the carbohydrates tested; however, compared to BB0 and BG0, the glucose content was almost twofold higher for LB0 (12.49 mg/kg) and more than threefold higher for LG0 (8.21 mg/kg), respectively (Table 3). Fermentation with different starter cultures was the main factor that affected the content of glucose (η2 = 0.487) and sucrose (η2 = 0.487) in LBs and led to a significant decrease in the content of these carbohydrates. In LBs, germination significantly reduced verbascose content and increased stachyose content, while fermentation significantly reduced the content of all the tested oligosaccharides. In all tested PBBs, the refrigerated storage period had the least effect on modifying the content of the analyzed carbohydrates.
According to Nkhata et al.27, the effect of germination on carbohydrates is largely dependent on the activation of hydrolytic and amylolytic enzymes; these enzymes decrease starch content and increase the content of simple sugars. Germination improves the digestibility of legumes by activating endogenous enzymes such as α-amylase. However, in the present study, germination led to a significant decrease in glucose content in the tested PBBs. The presence of glucose in the tested PBBs may result not only from the germination process used but also from other processing steps, including grinding. Ineffective grinding of the legumes may lead to a mass with a large particle size that will be retained on the sieves during the filtration process and will not be included in the beverage solution. This could reduce the concentration of dry matter in beverages and further result in a lower concentration of sugars28,29. Previous studies have shown that germinated BBs have a higher span and mean diameter d4.3 (span ≈ 2.24–2.35, d4.3 ≈ 76.8–84.2) than non-germinated BBs (span ≈ 1.90–2.00, d4.3 ≈ 38.2–47.0)30. This indicates the presence of larger particles or aggregates in the germinated beverage, which may directly result from the lower efficiency of the filtration process. The formation of protein aggregates is mainly observed in solutions prepared from legumes31. Germination leads to an increase in the availability of proteins32, which may result in more intensive formation of aggregates and, consequently, less effective grinding and filtration processes. Zahir et al.33 also observed the most intensive formation of protein aggregates in samples of germinated soybean preparations as compared to that in non-germinated ones.
TPC and antioxidant capacity
Free radicals, reactive oxygen species, and reactive nitrogen species are generated by our body through various endogenous systems when exposed to different physiochemical or pathological conditions34. The use of various processing techniques, including germination and fermentation, may increase the TPC and antioxidant capacity of legumes following a reduction in the anti-nutritional factors (ANFs) and the production of new components with antioxidant properties35,36. We are also aware that the total phenolic content theoretically, but not necessarily, correlates with the antioxidant activity, although this is widely accepted as a screening parameter. The Folin-Ciocalteu method to quantify TPC has some limitations. This test is sensitive to pH, temperature, and reaction time as well as to inorganic and nonphenolic organic compounds (including reducing sugars) that react with the Folin reagent, thereby underestimating the phenol content37.
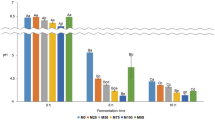
Figure 1 shows the TPC of the tested BBs (A) and LBs (B) and DPPH RSA of the tested BBs (C) and LBs (D). The results suggest that fermentation, choice of starter culture, germination, and storage conditions play crucial roles in determining the TPC of both BBs and LBs. These findings highlight the importance of these factors in modulating the antioxidant potential and potential health benefits of the tested products. The TPC of the base LB was twice as high as that of the base BB, which confirms the previous report that lentils naturally contain higher levels of phenolic compounds than beans38,39. Fermentation with the three different starter cultures was the primary factor that influenced the TPC of the tested PBBs (η2 = 0.652 for BBs and 0.651 for LBs). Fermentation with the starter culture ABY-3 (BB/BBG/LB/LBG102) was the most effective process. The present study showed that the refrigerated storage period significantly reduced the TPC in BBs, as indicated by the coefficient of determination (η2) value of 0.505. However, the refrigerated storage period did not affect the results obtained for LBs, thus implying that the phenolic content in LBs remained stable during refrigeration.
Total phenolic content of the tested BBs (A) and LBs (B) and DPPH radical scavenging activity of the tested BBs (C) and LBs (D). a,b,c,d,e,f,gMean values in columns denoted by different letters differ significantly (p ≤ 0.05). 1Description as in Table 1. All analyses were made in duplicate.
All the tested PBBs exhibited a relatively high DPPH scavenging capability (> 83%) (Fig. 1). The base BB and LB showed a similar ability to scavenge DPPH radicals (⁓85%). Fermentation with the three different starter cultures was the primary factor that influenced the DPPH RSA of the tested PBBs (η2 = 0.601 and 0.752 for BBs and LBs, respectively). Similar to the results obtained for TPC, fermentation with the starter culture ABY-3 (BB/BBG/LB/LBG102) was the most effective process. Germination significantly affected the obtained results only for the BBs (η2 = 0.285). Refrigerated storage had no effect on the DPPH RSA of all tested PBBs. This might be due to the presence of microorganisms and their metabolic activity, which also occurs during storage. LAB and bifidobacteria can produce bioactive peptides that increase the antioxidant capacity of the product40,41. Bioactive peptides are short amino acid sequences released from proteins by proteolysis, which are generated by enzymes produced by microorganisms42. Heydari et al.43 studied the effects of Iranian probiotic and commercial strains that included B. lactis, L. acidophilus, and L. casei on the functional properties of a water-soluble peptide extract. The tested microorganisms showed proteolytic activity during the 28-day cold storage period and significantly affected the formation of antioxidant, antimutagen and antibacterial peptides.
In the present study, the tested PBBs exhibited antioxidant properties derived from TPC and DPPH RSA. Compared to TPC, the DPPH RSA of the tested PBBs was more stable after the application of biotechnological processes and storage. This finding suggests that not only phenolic components but also other ingredients such as ascorbate, tocopherols, or carotenoids are responsible for the DPPH RSA of the studied PBBs44.
Fermentation was the primary factor that increased the TPC and DPPH RSA of the tested samples. Regardless of whether microbial fermentation is performed using fungi, yeasts, or bacteria, it affects the content of phenolic compounds in foods; moreover, metabolic activities are specific to the involved species or strains and depend on their array of enzymes. Among the fermented legumes, phenolic acid decarboxylase and esterase activities were reported in fermented cowpeas, lentils, and chickpeas36. In the present study, fermentation using the ABY-3 culture showed the greatest ability to increase the antioxidant properties of the tested beverages; this is because the culture contained the largest variety of microorganisms (L. acidophilus La-5, B. animalis subsp. lactis BB-12, S. thermophilus, and L. delbrueckii subsp. bulgaricus) among the tested starter cultures. Greater microbial diversity may present a broader enzyme array, which may increase the availability of antioxidant components, e.g., through the breakdown of ANFs11. In addition, the ABY-3 starter culture contains the strain B. animalis subsp. lactis BB-12, which is the world’s most documented probiotic Bifidobacterium45. These microorganisms produce a significant amount of bioactive peptides that may also exhibit antioxidant properties (e.g., iron-binding ferritin-like antioxidant protein)46. Previous studies also indicate that this strain has higher antioxidant activity than LAB47,48.
The effect of germination on the TPC and DPPH RSA of the tested PBBs varied depending on the analyzed raw material. Although these properties of BBGs and LBGs have not been previously investigated by other researchers, inconsistent results, depending on the tested raw material, were obtained for raw G and non-G legumes49,50,51. Therefore, the combination of germination and fermentation is the most beneficial approach to extend the effect on increasing the antioxidant properties of PBBs. This was also indicated by a previous study of Hubert et al.52, in which fermented soy germ extracts exhibited a higher inhibition effect against the superoxide anion radical, and lesser but significant DPPH radical scavenging effects compared to raw soy germ.
Total share of FA and FA profile
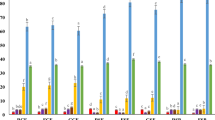
The content of FAs and the ratio between unsaturated fatty acids (UFAs) and saturated fatty acids (SFAs) are the important parameters to determine the nutritional value of fats53,54. Figure 2 and Supplementary Table S3 online shows the total share of UFAs and SFAs in the tested PBBs. UFAs dominated in all the tested PBBs, constituting 77.3–86.7% in BBs/BBGs and 74.1–80.3% in LBs/LBGs. Fermentation with the ABY-3 starter culture had a significant effect on the content of SFAs and UFAs in BBs/BBGs. Fermentation significantly increased the concentration of UFAs from 81.5% to 86.7% and simultaneously decreased the concentration of SFAs in BBG. In the remaining PBBs, fermentation, germination, and storage did not affect the total share of SFAs and UFAs.
Total share of unsaturated and saturated fatty acids in the tested BBs (A) and LBs (B). a,b,c,d,eMean values in the group of FA denoted by different letters differ significantly (p ≤ 0.05). 1Description as in Table 1. All analyses were made in duplicate.
In the FA profile of the tested PBBs, linolenic acid (C18:2 n-6c) was the dominant FA (Table 4). The other UFAs with a significant share in the tested PBBs were α-linolenic acid and oleic FAs. Only two SFAs, namely palmitic acid and stearic acid, had a significant share in the FA profile of the tested PBBs. The remaining FAs had a share of approximately 0.1% or less than that in the FA profile.
Fermentation with the ABY-3 starter culture was the primary factor that affected the FA profile of BBs and BBGs (η2 ≈ 0.306–0.929) (Table 4). Fermentation significantly modified the profile of n-3 and n-6 FAs by increasing the share of α-linolenic acid n-3 and reducing the share of linoleic acid n-6. Moreover, fermentation significantly reduced the content of palmitic acid in BBGs and reduced stearic acid content in BBs and BBGs. Germination significantly increased the share of only stearic acid in BBG (from 3.0 to 3.25%).
Both fermentation and germination showed a significant effect on the FA profile of the tested LBs and LBGs. Fermentation with the ABY-3 starter culture significantly reduced the share of stearic acid and oleic acid and increased the share of α-linolenic acid (Table 4). Germination significantly reduced the share of oleic acid and α-linolenic acid and increased the share of linoleic acid. The refrigerated storage period had no effect on the FA profile of all the tested PBBs.
UFAs dominated the FA profile of the tested PBBs, thus making beans and lentils suitable for nutritional applications. A similar trend of the dominance of UFAs in FA profiles was observed for nonfermented legume-based beverages, including BB18,55 and soy-based beverages56. In the present study, among the SFAs, only palmitic acid and stearic acid had a significant share, with palmitic acid being the predominant SFA; palmitic acid was also identified in raw beans and lentil seeds18,57. The FA profile in the fats of the legume seeds, however, differs considerably between the legume species and even between their varieties58. This is reflected directly by the FA profile of PBBs prepared from them.
During the production of legume-based beverages, it is beneficial to use processes that will contribute to increase the share of n-3 UFAs and/or reduce the share of SFAs in the FA profile. In the tested PBBs, the use of fermentation to modify the FA profile was more efficient than the use of germination. Fermentation using the ABY-3 starter culture significantly increased the share of α-linolenic acid and decreased the share of stearic acid in the FA profile of BBs/BBGs and LBs/LBGs. In all the tested PBBs, a multidirectional modification of the content of the remaining analyzed FAs was also observed. According to Adebo et al.59, the observed increase and decrease in these fat-related constituents after fermentation suggest selective lipase activities. While these lipolytic enzymes could have contributed to lipid dissociation and increased the extractability of fat-related constituents, the same enzymes could have also exerted selective reductive activities, perhaps using these fat-related components as carbon sources.
In the tested PBBs, germination mainly influenced the modification of the share of UFAs in the FA profile of LBs and LBGs by reducing the share of oleic and α-linolenic acids. The decrease in FAs could be due to hydrolysis during seed germination, where they were used to produce the necessary energy for biochemical and physicochemical modifications60. A significant modification of the UFAs in the FA profile during germination was also reported in raw lentil seeds60,61.
Positional distribution of FAs in TAGs
The intramolecular structure of TAGs in terms of the position of the FA chain in the glycerol backbone (sn1, sn2, and sn3 positions) influences the digestion and absorption of FAs62. Pancreatic lipase, which is responsible for the hydrolysis of TAGs, hydrolyzes the sn1 and sn3 positions of dietary TAGs, thereby producing free FAs and 2-monoglyceride. Structural rearrangement of 2-monoglycerides can occur during digestion, resulting in complete degradation into glycerol and free FAs63. In plants, TAGs are synthesized with structural specificity: SFAs can be found mostly in terminal positions, while UFAs are located at the sn-2 position64. There are very few scientific reports on the effect of biotechnological processes on the distribution of FAs in TAGs in food, and these reports are related to the use of lipids in human milk fat substitutes65.
Tables 5 and 6 show the positional distribution of sn2 FAs in TAGs and the positional distribution of sn1,3 FAs in TAGs in the tested PBBs, respectively. In all nonfermented PBBs, the highest share of palmitic acid was found at the sn2 position in TAGs. Among UFAs, oleic acid had the largest share of all sn2 FAs. Similarly, in the sn1,3 position in TAGs, PUFAs, i.e., linoleic acid and α-linolenic acid, had the highest share. Fermentation was the main factor that influenced the changes in the distribution of sn2 and sn1,3 FAs in TAGs. The application of fermentation significantly reduced the share of SFAs and monounsaturated fatty acids (MUFAs), increased the share of PUFAs in the sn2 position in TAGs, increased SFAs and MUFAs, and decreased PUFAs in the sn1,3 position in TAGs for BB/BBG/LB102. The exception was LBGs, where the share of PUFAs in the sn2 position in TAGs decreased for linoleic and α-linolenic acids, and consequently, the share of α-linolenic acid in the sn1,3 position in TAGs increased. Moreover, after the fermentation, previously absent stearic acid in BBs and BBGs and myristic acid in LBs and LBGs appeared among the sn2 and sn1,3 FAs in TAGs. The refrigerated storage period had no effect on FA distribution in TAGs in most of the tested PBBs.
To date, it remains unclear how fermentation using complex starter cultures affects the changes in the distribution of FAs in the TAGs of PBBs. Ziarno et al.18 analyzed the effect of fermentation of BBs on the positional distribution of FAs in TAGs by using monocultures of Lactobacillus. After fermentation, the BBs usually showed a lower share of palmitic and stearic acid in the sn2 position. The fermentation also increased the share of oleic acid in the sn2 position as compared to that in BBs obtained from germinated beans. Fermented beverages showed an average higher share of PUFAs (linoleic and α-linolenic acids) than that observed in the nonfermented PBBs. The present study showed similar results for SFAs and PUFAs.
The obtained results indicate that the fermentation of legume-based beverages increases the share of PUFAs in the sn2 position in TAGs and simultaneously increases the share of SFAs and MUFAs in the sn1,3 position in TAGs. This is beneficial from a nutritional point of view, as SFAs and MUFAs will be first hydrolyzed by pancreatic lipase and separated from TAGs. These FAs will be less efficiently absorbed in the intestine because they can react with free calcium ions to form insoluble calcium salts, which are then excreted in the feces66. PUFAs located mainly at the sn2 position in TAGs will be mostly absorbed as monoacylglycerols. PUFAs are referred to as dietary essential FAs because humans cannot synthesize these molecules; therefore, they must be supplied with the diet67.
Conclusions
The development of legume-based beverages is essential to provide consumers with PBBs with a high nutritional value, particularly those with a protein content similar to milk. Although this segment of PBBs is still poorly researched, previous studies indicate limitations related to their consumption, such as the presence of ANFs or barriers related to taste and texture. The present study demonstrated that the use of natural biotechnological processes in the production of newly developed beverages made from beans and lentils enables to increase their nutritional properties. The use of germination and fermentation improved the antioxidant properties, modified the oligosaccharide content, and altered the profile and positional distribution of FAs in TAGs of the tested PBBs. Importantly, the tested beverages were a good matrix for the fermentation process using LAB and bifidobacteria, including the probiotic species.
Based on the findings of the present study, we recommend that the designing of innovative legume-based beverages should include the use of fermentation and germination processes that will allow to obtain products that serve as carriers of probiotic bacteria and have improved nutritional properties. Moreover, it is recommended to use multispecies starter cultures containing both LAB and bifidobacteria in the fermentation of legume-based beverages, which enables a more efficient process. These products can directly meet the market demand for high-quality plant products as a substitute for animal products. Future research should focus on analyzing the properties of PBBs derived from other legumes and conducting a broad assessment of their sensory properties, which may be the most important factor limiting the consumption of PBBs by consumers. Moreover, the presented study considered selected health-promoting and functional properties of BBs and LBs as plant products with high nutritional value. These beverages are poorly described in the literature, so we recommend that future research should focus on the analysis of their other properties, in particular the quantity and quality of proteins and amino acids. This will allow for an in-depth analysis of these products also in terms of their suitability as analogues of cow's milk.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gasbarrini, G., Bonvicini, F. & Gramenzi, A. Probiotics history. J. Clin. Gastroenterol. 50, 116–119. https://doi.org/10.1097/MCG.0000000000000697 (2016).
Benincasa, P., Falcinelli, B., Lutts, S., Stagnari, F. & Galieni, A. Sprouted grains: A comprehensive review. Nutrients 11, 421. https://doi.org/10.3390/nu11020421 (2019).
Xiang, H., Sun-Waterhouse, D., Waterhouse, G., Cui, C. & Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 8, 203–243. https://doi.org/10.1016/j.fshw.2019.08.003 (2019).
Miyahira, R. F., Lopes, J. & Antunes, A. E. The use of sprouts to improve the nutritional value of food products: A brief review. Plant Foods Hum. Nutr. 76, 143–152. https://doi.org/10.1007/s11130-021-00888-6 (2021).
Chaturvedi, S. & Chakarborty, S. Review on potential non-dairy synbiotic beverages: A preliminary approach using legumes. Int. J. Food Sci. Technol. 56, 2068–2077. https://doi.org/10.1111/ijfs.14779 (2020).
Cichońska, P. & Ziarno, M. Legumes and legume-based beverages fermented with lactic acid bacteria as a potential carrier of probiotics and prebiotics. Microorganisms 10, 91. https://doi.org/10.3390/microorganisms10010091 (2021).
Reyes-Jurado, F. et al. Plant-based milk alternatives: Types, processes, benefits, and characteristics. Food Rev. Int. https://doi.org/10.1080/87559129.2021.1952421 (2021).
Fructuoso, I. et al. An overview on nutritional aspects of plant-based beverages used as substitutes for cow’s milk. Nutrients 13, 2650. https://doi.org/10.3390/nu13082650 (2021).
Lopes, M. et al. Legume beverages from chickpea and lupin, as new milk alternatives. Foods 9, 1458. https://doi.org/10.3390/foods9101458 (2020).
Nawaz, M. A., Tan, M., Øiseth, S. & Buckow, R. An emerging segment of functional legume-based beverages: A review. Food Rev. Int. 38, 1064–1102. https://doi.org/10.1080/87559129.2020.1762641 (2022).
Sakandar, H. A. et al. Impact of fermentation on antinutritional factors and protein degradation of legume seeds: A review. Food Rev. Int. https://doi.org/10.1080/87559129.2021.1931300 (2021).
Rasika, D. M. et al. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 38, 8–20. https://doi.org/10.1016/j.cofs.2020.10.025 (2021).
Penha, C. B., De Paola Santos, V., Spernaza, P. & Kurozawa, L. E. Plant-based beverages: Ecofriendly technologies in the production process. Innov. Food Sci. Emerg. Technol. 72, 102760. https://doi.org/10.1016/j.ifset.2021.102760 (2021).
Ohanenye, I. C., Tsopmo, A., Ejike, C. & Udenigwe, C. C. Germination as a bioprocess for enhancing the quality and nutritional prospects of legume proteins. Trends Food Sci. Technol. 101, 213–222. https://doi.org/10.1016/j.tifs.2020.05.003 (2020).
Ziarno, M., Zareba, D., Maciejak, M. & Veber, A. L. The impact of dairy starter cultures on selected qualitative properties of functional fermented beverage prepared from germinated White Kidney Beans. J. Food Nutr. Res. 2, 167–176 (2019).
Zhao, D. & Shah, N. P. Antiradical and tea polyphenol-stabilizing ability of functional fermented soymilk–tea beverage. Food Chem. 158, 262–269. https://doi.org/10.1016/j.foodchem.2014.02.119 (2014).
Eggers, L. F. & Schwudke, D. Liquid extraction: Folch. In Encyclopedia of Lipidomics (ed. Wenk, M. R.) 1–6 (Springer Science + Business Media, 2016).
Ziarno, M., Bryś, J., Parzyszek, M. & Veber, A. Effect of lactic acid bacteria on the lipid profile of bean-based plant substitute of fermented milk. Microorganisms 8, 1348. https://doi.org/10.3390/microorganisms8091348 (2020).
Shafiee, G. et al. Combined effects of dry matter content, incubation temperature and final pH of fermentation on biochemical and microbiological, characteristics of probiotic fermented milk. Afr. J. Microbiol. Res. 4, 1265–1274 (2010).
Sfakianakis, P. & Tzia, C. Conventional and innovative processing of milk for yogurt manufacture; development of texture and flavor: A review. Foods 3, 176–193. https://doi.org/10.3390/foods3010176 (2014).
Fazilah, N. F., Ariff, A. B., Khayat, M. E., Rios-Solis, L. & Halim, M. Influence of probiotics, prebiotics, synbiotics and bioactive phytochemicals on the formulation of functional yogurt. J. Funct. Foods 48, 387–399. https://doi.org/10.1016/j.jff.2018.07.039 (2018).
Adamberg, S. et al. Survival and synergistic growth of mixed cultures ofbifidobacteria and lactobacilli combined with prebioticoligosaccharides in a gastrointestinal tract simulator. Microb. Ecol. 25, 23062. https://doi.org/10.3402/mehd.v25.23062 (2014).
Pokusaeva, K., Fitzgerald, G. F. & van Sinderen, D. Carbohydrate metabolism in bifidobacteria. Genes Nutr. 6, 285–306. https://doi.org/10.1007/s12263-010-0206-6 (2011).
Heller, K. J. Probiotic bacteria in fermented foods: Product characteristics and starter organisms. Am. J. Clin. Nutr. 73, 374–379. https://doi.org/10.1093/ajcn/73.2.374s (2001).
Sanchez-Chino, X., Jimenez-Martinez, C., Davila-Ortiz, G., Alvarez-Gonzalez, I. & Madrigal-Bujaidar, E. Nutrient and nonnutrient components of legumes, and its chemopreventive activity: A review. Nutr. Cancer 67, 401–410. https://doi.org/10.1080/01635581.2015.1004729 (2015).
Kannan, U., Sharma, R., Gangola, M. P. & Chibbar, R. N. Improving grain quality in pulses: Strategies to reduce raffinose family oligosaccharides in seeds. Ekin J. Crop Breed. Genetic. 4, 70–88 (2018).
Nkhata, S. G., Ayua, E., Kamau, E. H. & Shingrio, J. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 6, 2446–2458. https://doi.org/10.1002/fsn3.846 (2018).
Kinnarinen, T., Tuunila, R. & Häkkinen, A. Reduction of the width of particle size distribution to improve pressure filtration properties of slurries. Miner. Eng. 102, 68–74. https://doi.org/10.1016/j.mineng.2016.12.009 (2017).
Vishwanathan, K. H., Singh, V. & Subramanian, R. Wet grinding characteristics of soybean for soymilk extraction. J. Food Eng. 106, 28–34. https://doi.org/10.1016/j.jfoodeng.2011.04.002 (2011).
Cichońska, P., Domian, E. & Ziarno, M. Application of optical and rheological techniques in quality and storage assessment of the newly developed colloidal-suspension products: Yogurt-type bean-based beverages. Sensors 22, 8348. https://doi.org/10.3390/s22218348 (2022).
Tang, C.-H., Chen, L. & Ma, C.-Y. Thermal aggregation, amino acid composition and in vitro digestibility of vicilin-rich protein isolates from three Phaseolus legumes: A comparative study. Food Chem. 113, 957–963. https://doi.org/10.1016/j.foodchem.2008.08.038 (2009).
Ohanenye, I. C. et al. Legume seed protein digestibility as influenced by traditional and emerging physical processing technologies. Foods 11, 2299. https://doi.org/10.3390/foods11152299 (2022).
Zahir, M., Fogliano, V. & Capuano, E. Soybean germination limits the role of cell wall integrity in controlling protein physicochemical changes during cooking and improves protein digestibility. Food Res. Int. 143, 110254. https://doi.org/10.1016/j.foodres.2021.110254 (2021).
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Phcog. Rev. 4, 118–126. https://doi.org/10.4103/0973-7847.70902 (2010).
Vaz Patto, M. C. et al. Achievements and challenges in improving the nutritional quality of food legumes. Crit. Rev. Plant Sci. 34, 105–143. https://doi.org/10.1080/07352689.2014.897907 (2015).
Verni, M., Verardo, V. & Rizello, C. G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 8, 362. https://doi.org/10.3390/foods8090362 (2019).
Box, J. D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 17, 511–525. https://doi.org/10.1016/0043-1354(83)90111-2 (1983).
Zhao, Y., Du, S., Wang, H. & Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 152, 462–466. https://doi.org/10.1016/j.foodchem.2013.12.006 (2014).
Djordjevic, T. M., Šiler-Marinkovic, S. S. & Dimitrijevic-Brankovic, S. I. Antioxidant activity and total phenolic content in some cereals and legumes. Int. J. Food Prop. 14, 175–184. https://doi.org/10.1080/10942910903160364 (2011).
Amiri, S., Moghanjougi, Z. M., BarI, M. R. & Khaneghah, A. M. Natural protective agents and their applications as bio-preservatives in the food industry: An overview of current and future applications. Ital. J. Food Sci. 33, 55–68. https://doi.org/10.15586/ijfs.v33iSP1.2045 (2021).
Rahmawati, I. S. & Suntornsuk, W. Effects of fermentation and storage on bioactive activities in milks and yoghurts. Procedia Chem. 18, 53–62. https://doi.org/10.1016/j.proche.2016.01.010 (2016).
Chaves-López, C. et al. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 42, 117–121. https://doi.org/10.1016/j.fm.2014.03.005 (2014).
Heydari, S., Hosseini, S. E., Mortazavian, A. M. & Taheri, S. Extraction of bioactive peptides produced in probiotic yoghurt and determination of their biological activities. Int. Dairy J. 139, 105544. https://doi.org/10.1016/j.idairyj.2022.105544 (2023).
Kebede, M. & Admassu, S. Application of antioxidants in food processing industry: Options to improve the extraction yields and market value of natural products. Adv. Food Technol. Nutr. Sci. 5, 38–49. https://doi.org/10.17140/AFTNSOJ-5-155 (2019).
Jungersen, M. et al. The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12®. Microorganisms 2, 92–110. https://doi.org/10.3390/microorganisms2020092 (2014).
Gilad, O., Viborg, A. H., Stuer-Lauridsen, B. & Jacobsen, S. The extracellular proteome of Bifidobacterium animalis subsp. lactis BB-12 reveals proteins with putative roles in probiotic effects. Proteomics 11, 2503–2514. https://doi.org/10.1002/pmic.201000716 (2011).
Okoń, A., Stadnik, J. & Dolatowski, Z. J. Effect of Lactobacillus acidophilus Bauer and Bifidobacterium animalis ssp. lactis BB12 on proteolytic changes in dry-cured loins. Food Sci. Biotechnol. 26, 633–641. https://doi.org/10.1007/s10068-017-0076-4 (2017).
Bujna, E., Farkas, N. A., Tran, A. M., Dam, M. S. & Nguyen, Q. D. Lactic acid fermentation of apricot juice by mono- and mixed cultures of probiotic Lactobacillus and Bifidobacterium strains. Food Sci. Biotechnol. 27, 633–641. https://doi.org/10.1007/s10068-017-0269-x (2018).
Chon, S. Total polyphenols and bioactivity of seeds and sprouts in several legumes. Curr. Pharm. Des. 19, 6112–6124. https://doi.org/10.2174/1381612811319340005 (2013).
MinYing, C., Azlan, A., Al-Sheraji, S. H., Hassan, F. A. & Prasad, K. N. Antioxidant activities and total phenolic content in germinated and non-germinated legume extracts following alkaline-acid hydrolysis. Pak. J. Nutr. 12, 1036–1041. https://doi.org/10.3923/pjn.2013.1036.1041 (2013).
Khang, D. T., Dung, T. N., Elzaawely, A. A. & Xuan, T. D. Phenolic profiles and antioxidant activity of germinated legumes. Foods 5, 27. https://doi.org/10.3390/foods5020027 (2016).
Hubert, J., Berger, M., Napveu, F., Paul, F. & Dayde, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 109, 709–721. https://doi.org/10.1016/j.foodchem.2007.12.081 (2008).
Baum, S. J. et al. Fatty acids in cardiovascular health and disease: A comprehensive update. J. Clin. Lipidol. 6, 216–234. https://doi.org/10.1016/j.jacl.2012.04.077 (2012).
Kostik, V., Memeti, S. & Bauer, B. Fatty acid composition of edible oils and fats. JHED 4, 112–116 (2013).
Aydar, E. F., Metdinc, Z., Demiracan, E., Cetinkaya, S. K. & Ozcelik, B. Kidney bean (Phaseolus vulgaris L.) milk substitute as a novel plant-based drink: Fatty acid profile, antioxidant activity, in-vitro phenolic bio-accessibility and sensory characteristics. Innov. Food Sci. Emerg. Technol. 83, 103254. https://doi.org/10.1016/j.ifset.2022.103254 (2023).
Martinez-Padilla, E. et al. In vitro protein digestibility and fatty acid profile of commercial plant-based milk alternatives. Foods 9, 1784. https://doi.org/10.3390/foods9121784 (2020).
Gharibzahedi, S. M., Mousavi, S. M., Jafari, S. M. & Faraji, K. Proximate composition, mineral content, and fatty acids profile of two varieties of lentil seeds cultivated in Iran. Chem. Nat. Compd. 47, 976–978. https://doi.org/10.1007/s10600-012-0119-2 (2012).
Grela, E. R., Samolińska, W., Kiczorowska, B., Klebaniuk, R. & Kiczorowski, P. Content of minerals and fatty acids and their correlation with phytochemical compounds and antioxidant activity of leguminous seeds. Biol. Trace Elem. Res. 180, 338–348. https://doi.org/10.1007/s12011-017-1005-3 (2017).
Adebo, J. A. et al. Fermentation of cereals and legumes: Impact on nutritional constituents and nutrient bioavailability. Fermentation 8, 63. https://doi.org/10.3390/fermentation8020063 (2021).
Pal, R. S. et al. Effect of dehulling, germination and cooking on nutrients, anti-nutrients, fatty acid composition and antioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 54, 909–920. https://doi.org/10.1007/s13197-016-2351-4 (2016).
Alkaltham, M. S., Özcan, M. M., Uslu, N., Salamatullah, A. M. & Hayat, K. Changes in antioxidant activity, phenolic compounds, fatty acids, and mineral contents of raw, germinated, and boiled lentil seeds. J. Food Sci. 87, 1639–1649. https://doi.org/10.1111/1750-3841.16099 (2022).
Dima, C., Assadpour, E., Dima, S. & Jafari, S. M. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 19, 954–994. https://doi.org/10.1111/1541-4337.12547 (2020).
Golding, M. & Wooster, T. J. The influence of emulsion structure and stability on lipid digestion. Curr. Opin. Colloid Interface Sci. 15, 90–101. https://doi.org/10.1016/j.cocis.2009.11.006 (2010).
Gerbig, S. & Takats, Z. Analysis of triglycerides in food items by desorption electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 24, 2186–2192. https://doi.org/10.1002/rcm.4630 (2010).
Zhang, L., Chu, M., Zong, M., Yang, J. & Lou, W. Facile and green production of human milk fat substitute through rhodococcus opacus fermentation. J. Agric. Food Chem. 68, 9368–9376. https://doi.org/10.1021/acs.jafc.0c03185 (2020).
Bryś, J. & Wirkowska, M. Knowledge of the structure of triacylglycerols in designing structured lipids. Postępy Techniki Przetwórstwa Spożywczego 2, 86–89 (2010).
Lee, J. M., Lee, H., Kang, S. & Park, W. J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 8, 23. https://doi.org/10.3390/nu8010023 (2016).
Acknowledgements
White kidney beans “Piękny Jaś Karłowy” were purchased from Lestello Sp. z o.o., Cmolas, Poland and brown lentils were purchased from Natural Expert, Bialystok, Poland. All procedures for using the seeds of the plants have been carried out in accordance with the institutional, national, and international guidelines and laws. We would like to thank Paulina Capek, Iwona Puton, Karolina Szczurowska and Katarzyna Czarniak for their participation in laboratory analyses.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.C. and M.Z.; methodology, P.C., J.B. and M.Z.; data curation, P.C.; formal analysis, P.C., J.B. and M.Z.; investigation, P.C. and J.B.; writing—original draft preparation P.C.; writing—review & editing, J.B. and M.Z.; validation, P.C.; project administration, P.C.; Supervision, M.Z. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cichońska, P., Bryś, J. & Ziarno, M. Use of natural biotechnological processes to modify the nutritional properties of bean-based and lentil-based beverages. Sci Rep 13, 16976 (2023). https://doi.org/10.1038/s41598-023-44239-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44239-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.