Abstract
Neolithic farming and animal husbandry were first developed in the Near East ~ 10,000 BCE and expanded westwards, reaching westernmost Iberia no later than 5500 BCE. It resulted in major social, cultural, economic and dietary changes. Yet, the impact of this change on human mandibular morphology in Iberia is yet to be assessed, which is regrettable because mandible form is impacted by population history and diet. In this study we used Mesolithic to Chalcolithic Iberian samples to examine the impact of this transition on mandibular morphology. We also compared these samples with a Southern Levantine Chalcolithic population to assess their relationship. Lastly, we assessed dental wear to determine if the morphological differences identified were related to the material properties of the diet. We found differences between samples in mandibular shape but not size, which we attribute to contrasting population histories between Mesolithic and later populations. Some differences in the severity of dental wear were also found between Mesolithic and later Iberian samples, and smaller between the Mesolithic Iberians and southern Levantines. Little relationship was found between wear magnitude and mandibular shape. Altogether, our results show that the Mesolithic–Neolithic Iberian transition resulted in a meaningful change in mandibular morphology, which was likely driven more by population history than by dietary change.
Similar content being viewed by others
Introduction
Neolithic farming and animal husbandry were first developed in the Near East about 9000–10,000 BCE1 and subsequently expanded throughout Europe via two main routes: via the Danube into central Europe, and via the Mediterranean into Southern and Western Europe2,3,4,5,6,7. Ancient DNA (aDNA) studies have shown that the expansion of this new mode of subsistence was associated with demic diffusion of Near Eastern populations and their flocks that reached Western Iberia (i.e., modern-day Portugal and Galicia) no later than ~ 5500 BCE2,3,4,5,6,8,9,10,11. This is evident since aDNA studies show marked genetic discontinuity between Iberian Mesolithic foragers and the Neolithic agro-pastoralist populations, despite some level of admixture12,13,14,15,16.
The transition from Mesolithic foraging to Neolithic agro-pastoralism also involved profound dietary changes, including the shift from a mechanically more demanding diet in the Mesolithic to a softer diet in the Neolithic and later periods17,18. This change in the material properties of foodstuffs has been supported by studies of skeletal morphology from several geographic regions documenting that Mesolithic skulls were generally larger, more robust and differently shaped than Neolithic specimens19,20,21,22,23. Studies of dental wear magnitude in western Iberia have also shown that this was more severe in Mesolithic than in Neolithic and Chalcolithic populations, providing direct evidence of decreased masticatory demands from the Neolithic onwards24,25. Elsewhere, differences in dental microwear have also been detected between foragers, farmers and pastoralists26,27,28. Stable isotope-based research in this region has reported a transition from diverse Mesolithic diets (reflecting the exploitation of several ecological contexts) with a meaningful component of marine foodstuffs, to less diverse Neolithic diets largely dominated by terrestrial products in which marine products, despite consumed, were less relevant24,29,30,31,32,33. Lastly, recent metagenomic analyses have also shown changes in the oral microbiome following the adoption of Neolithic agro-pastoralism in the Central-South Italian Peninsula34. Such dietary changes have been shown to result in changes in dental disease patterns in different regions35,36,37,38,39,40.
Previous continental scale studies have shown that diachronic change in cranial morphology across Europe and the Levant is generally consistent with the archaeological record and aDNA results indicative of population movement as well as cultural diffusion41,42. Yet such studies are limited in the context of Iberia because they do not include post-Mesolithic specimens, thus precluding analysis of morphological change during the Mesolithic-Neolithic transition in the Peninsula. The only study on Mesolithic and Neolithic crania specific to Iberia is that of Jackes, Lubell and Meiklejohn43, who concluded that their findings did not support hypotheses of population discontinuity during this transition. However, these studies focused on cranial measurements and did not include mandibular form. Mandibular morphology has been shown to relate to both population history19,44,45 and diet19,20,21,22,23, and thus both population and dietary changes should impact Iberian mandibular morphology. Yet, to date, there is only one study on this topic46. In that study, the authors found differences in mandibular shape between Iberian Mesolithic foragers and Chalcolithic agro-pastoralists, but could not assess the timing of the changes identified, nor the extent to which they reflected demic change as opposed to dietary change.
Here we address these issues using Geometric Morphometrics (GM) to examine the impact of the Mesolithic–Neolithic transition on Iberian mandibular morphology by comparing local Mesolithic foragers, Neolithic and Chalcolithic agro-pastoralists, and southern Levantine Neolithic and Chalcolithic agro-pastoralists. We also scored dental wear, to assess the contribution of function to the observed morphological differences. Based on aDNA studies and the relationship between mandibular morphology and population history (see above), we expect significant differences between Mesolithic Iberians and Chalcolithic/Neolithic southern Levantines, and test this against a null hypothesis of no differences between these populations. We further hypothesise that post-Mesolithic Iberians are intermediate between the two former populations. Last, we examine the relationship between dental wear and mandibular shape and (considering the relationship between mandibular morphology and diet) expect to find a significant relationship between the two.
Results
We found no significant differences in mandibular size between groups (Fig. 1; H(5) = 6.5186, p = 0.259) but did find significant differences between them in shape. Using PERMANOVA we found that the scores from the first 29 PCs (explaining ~ 95% of the total variance) showed statistically significant shape differences (p < 0.0001) between the different groups. Pairwise comparisons revealed highly significant differences between Mesolithic Iberia and all other groups (p < 0.01) except for the Late Neolithic—Chalcolithic Iberian sample, where the results were borderline (p = 0.051), while the Levantine Chalcolithic group was significantly different from all the Iberian groups (Table 1).
Visual assessment of the distribution of individuals along PC1 (16.37% of the variance) and PC2 (10.49% of the variance) shows some overlap between the Mesolithic Iberian and the Chalcolithic Levantine specimens (Fig. 2, SI Fig. 1). Nevertheless, these two groups are significantly different (SI Fig. 2). Iberian post-Mesolithic specimens fall between the Iberian Mesolithic and Levantine Chalcolithic groups, overlapping with both and showing no statistically significant differences from either in these two PCs (Fig. 2, SI Fig. 2). Morphologically, specimens located in the positive end of PC1 have wider rami and a more projecting anterior alveolar region. Specimens which are in the positive end of PC2 have more inferiorly positioned coronoid processes, more flexed posterior margins of the ramus and less projecting gonia. Visual assessment of Fig. 2 also suggests more diversity in the Chalcolithic Iberian sample than in others. This assessment is supported by the analysis of shape disparity, which shows that the within-group Procrustes distances are greater in Chalcolithic Iberia than in all other groups (Mesolithic Iberia = 0.0039, Neolithic Iberia = 0.0042, Late Neolithic—Chalcolithic Iberia = 0.0030, Chalcolithic Iberia = 0.0062, Chalcolithic Levant = 0.0034; Table 2).
PCA of mandibular shape. Individuals are grouped according to chronology and geographic origin. The Mesolithic Iberian and Chalcolithic Levant groups are morphologically distinct, despite some overlap. The post-Mesolithic Iberian specimens are intermediate between the two extreme previous groups (i.e., Mesolithic Iberia and Chalcolithic Levant), overlapping with both. The mandible insets represent the morphology of the specimens at the extremes of the PC axes (e.g., the mandible at the bottom left corner represents the morphology of a hypothetical specimen located at the negative end of PCs 1 and 2).
The total number of teeth in several of the samples was small primarily because of post-mortem loss of anterior teeth (Table SI 1), but comparison of all tooth types indicated that tooth wear was heavier in the Iberian Mesolithic group than in the more recent Iberian samples (Fig. 3, SI Fig. 3). More meaningful comparisons of the magnitude of tooth wear were made on the first and second molars, where samples sizes were larger. The results showed that the magnitude of wear in first molars was significantly greater in the Iberian Mesolithic than in most other samples (SI Fig. 3). In the second molar, differences in dental wear followed the same pattern, but statistically significant differences were only present between the Iberian Mesolithic and Chalcolithic samples from Iberia and the Levant (SI Fig. 3).
Dental wear magnitude, scored according to B.H. Smith 47, across Mesolithic, Neolithic and Chalcolithic groups. Results for the Iberian and Levantine samples are shown separately. In the Levantine sample there are no Mesolithic or Late Neolithic—Chalcolithic specimens, and only one Neolithic specimen.
The differences in severity of wear of paired first and second molars is shown in Fig. 4. The wear scores plotted for the different groups varied, with few Iberian Neolithic or Chalcolithic first molars scoring 5 or more. This contrasted with the Iberian Mesolithic and Levantine Chalcolithic molars where more severe wear scores were common. This was reflected in the slope of the regression lines that were plotted. These were steepest in the Iberian and Levantine Chalcolithic samples. However, no statistically significant differences were found between groups (SI Fig. 4).
Regressing mandibular shape (as assessed by the scores of the first 29 PCs) against first and second molar dental wear magnitude scores yields very low R2 values and non-significant p values in most cases (Table SI 2), thus revealing little impact of masticatory function (as assessed by wear magnitude) on mandibular morphology.
Discussion
The results obtained show no difference in mandibular size between samples, but significant shape differences between Mesolithic Iberian and almost all other populations, as well as between the Levant Chalcolithic and all other groups when considering the PCs explaining ~ 95% of the variance. The first two PCs (~ 27% variance) show that the Iberian Neolithic to Chalcolithic samples overlap with both the Iberian Mesolithic and Levantine Chalcolithic samples (which are statistically significantly different from each other) and show no significant differences when compared to any of all other populations. This intermediate position is expected given the relationship between mandibular morphology and population history19,44,45, and accords with the aDNA findings demonstrating diffusion of Neolithic Near Eastern populations into Iberia and some degree of subsequent admixture with the local pre-existing population2,3,4,8,15,16. Overall, these results confirm our prediction of rejection of the null hypothesis of no differences between Mesolithic Iberia and the Chalcolithic Levant, and that post-Mesolithic Iberians are morphologically intermediate between these groups.
Moreover, our results for severity of dental wear in molars show highly significant differences between the Iberian Mesolithic, more recent Iberian and the Levantine samples. This is most severe in the Iberian Mesolithic followed by the Levantine Chalcolithic and least severe in the Iberian Neolithic and Chalcolithic sample. Because dental wear relates to the material properties of foodstuffs24,47,48,49,50,51,52,53,54, this suggests limited differences in the material properties of the diets between these two former populations. Interestingly, visual assessment of morphological differences across specimens along PC1 and 2 suggests that the Iberian Mesolithic specimens are generally more gracile than the Chalcolithic Levant mandibles (Fig. 2) despite the heavier dental wear of the Iberian Mesolithic sample that would be expected to affect biomechanical adaptation of the mandible55,56,57,58,59,60,61. Moreover, and contrary to our original prediction, regressing PC scores against dental wear revealed very little relationship between the two, thus showing that dental wear magnitude only explains a very minor proportion of shape variance. Altogether this suggests that population history (rather than masticatory mechanics) plays the most important role on the differences seen in mandibular morphology in our study.
Several previous studies support gradual dietary changes throughout the Neolithic and Chalcolithic, i.e., after the introduction of farming (and therefore of different material properties of foodstuffs). Godinho, et al.25 found evidence of decreased dental wear in Iberia in the transition from the Neolithic to the Chalcolithic, possibly related to agricultural intensification. Dietary stable isotope analysis in Iberia also supports a gradual change from the early Neolithic to the Chalcolithic period which was related to agricultural intensification30. Lastly, Quagliariello, et al.34 found evidence of changes in the oral microbiome during the Neolithic—Chalcolithic transition that they attributed to changes in diet. Our results show overlap of the Iberian Neolithic to Chalcolithic samples and so do not detect a gradual change in mandibular form throughout this chronological span. This may be consistent with the greater impact of population history on mandibular morphology. Although our results for dental wear do not detect meaningful changes in dental wear between the Neolithic and the Chalcolithic, the dental sample is very small (in some cases n = 1), precluding well supported considerations.
Interestingly, we detected larger morphological variance (i.e., disparity) in the Iberian Chalcolithic than in other samples. This finding is of particular relevance because of the presence of dental and skeletal traits in this period in Iberia that are more common in African populations62,63. Moreover, aDNA studies show some contact between Chalcolithic Iberians and North Africans15, and the presence of raw materials originating in regions as distant as Asia and North Africa relate to the expansion of Chalcolithic trading networks64,65,66,67,68,69. Higher levels of mobility during this period are also reflected in the presence of non-local individuals in multiple regions70,71,72. We thus hypothesize that the large morphological disparity detected in the Iberian Chalcolithic mandibles may relate to increased contact with exogenous populations.
The population and dietary changes in the Mesolithic–Neolithic transition are also associated with other socio-economic-biological changes17. Specifically, the Mesolithic forager economy is typically associated with high levels of mobility that are reflected in high bending strength of the femur and tibia73,74,75,76. Interestingly, Iberian Mesolithic populations are reported to have limited bending strength of the lower limb when compared to later populations from the Bronze Age77. While this contrasts with the overall pattern of lower limb morphology in this timeframe, these are late Mesolithic populations that are already somewhat sedentary and occupied an area with low topographic relief, which is reflected in the cross-sectional anatomy of the bones of the lower limb24,77.
Despite our interpretation that (i) mandibular shape differences between Iberia and the southern Levant are likely mostly due to contrasting population histories, and (ii) the overlap shown by the two most relevant PCs between the Iberian Neolithic to Chalcolithic group to all others is probably related to further contacts over time in the wake of increased trade and population movements, the following caveats and limitations should be considered.
The single Neolithic Levantine specimen from Abu Gosh that was used in this study dates to the final stage of the Neolithic period, so it is not surprising that it falls within the range of variation of the Levantine Chalcolithic sample. In this it conforms to results of previous studies that showed no differences between southern Levantine Neolithic and Chalcolithic mandibular morphology21. Moreover, aDNA studies78 have shown that Neolithic and Chalcolithic southern Levantine populations are directly related. Thus, although earlier Neolithic southern Levantine populations would be better suited to examine the relationship between Neolithic-Chalcolithic Iberian mandibular morphology and population admixture between Mesolithic Iberians and migrating Near Eastern populations, the southern Levantine Chalcolithic sample appears a generally reliable proxy.
This study compares Iberian and southern Levantine samples. aDNA studies have shown that Iberian Neolithic-Chalcolithic populations are closer to Anatolian and European Neolithic populations than to those from the southern Levant7,15,79. This reflects the “dilution” of the initial Near Eastern phenotype through admixture with other Mediterranean populations over time and space as populations spread westward. Thus, although a relationship between the recent Prehistoric southern Levantine and Iberian samples is to be expected, it is impacted by the complexity of the westward expansion of the Neolithic to Europe. Thus, future studies should expand the non-Iberian sample and include more southern Levantine specimens from the Neolithic and Chalcolithic to ensure there are no meaningful differences between these chronologically distinct groups, and that the shape differences detected in this study between the Iberian and southern Levantine samples do not result from sampling bias.
Despite these caveats for future research, our study documents significant mandibular shape differences between the Iberian Mesolithic and the southern Levant Chalcolithic, with the Iberian Neolithic to Chalcolithic samples occupying an intermediate position between them. Further, we found little relationship between mandibular shape and dental wear, limited differences in dental wear (and so function) between the Mesolithic Iberians and the Chalcolithic southern Levantine groups, and larger differences relative to post-Mesolithic Iberians. Altogether, this is consistent with demic diffusion of Near Eastern Neolithic populations. Thus, admixture with incoming Neolithic populations may have contributed to the extent and pattern of changes, as well as increased variation, seen in the teeth and jaws of Iberian Neolithic and Chalcolithic populations. Moreover, dietary differences appear to have had limited impact on the observed morphological differences across our samples.
Materials and methods
Sample
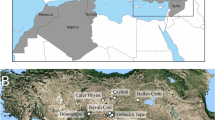
In this study we examined 101 human mandibles originating from southwestern Iberia (central and southern Portugal) and the southern Levant (Judean desert), that chrono-culturally span the Mesolithic to Chalcolithic periods (Fig. 5 and Table 3). Only adult specimens were selected (i.e., specimens at least 18 years old). The reason is that mandibular form changes dramatically throughout growth and development80,81, and so it is critical to ensure that age-related morphological differences do not overshadow group-related differences, which were the focus of this study. Mandibular morphology is also impacted by sexual dimorphism82,83,84,85,86. Yet, morphological based sex estimation is most reliable when using the hip bones and the full skull87,88 (which is not possible in most cases with these samples; see details below). Previous studies using identified collections report correct classification rates of as low as 68%85,86 when estimating sex based on mandibles alone, showing potential limitations of this approach. Moreover, morphological sex differences are not explained exclusively by size (both isometric and allometric) differences83,89,90, limiting the efficacy of removing size related shape differences to exclude the effects of sexual dimorphism from ensuing analyses. Thus, although morphological based sex estimation was undertaken using only the mandibles, this data was not included because it is not conclusive and so may bias the results of the study.
Age at death assessment
Age at death was estimated based on dental development and eruption using the standards of AlQahtani, Hector and Liversidge93. To that end, macroscopic observation of these parameters was supplemented by Computed Tomography (CT) scan-based assessment of tooth growth and development whenever possible (see below), allowing observation of non-erupted teeth and roots. No other age-at-death proxies were used because the nature of the samples does not allow conclusive identification and analysis of skeletal elements from the same individual other than the mandible. This is because several Mesolithic samples were affected by post-excavation mixing94 and the post-Mesolithic Iberian and Levantine samples originated from collective, commingled funerary contexts95,96,97,98,99. Alternatively, tooth wear-based estimation could have been used due to the relationship between wear magnitude and age54,100,101. However, since it is also impacted by diet, and in some cases paramastication, it is not a reliable age-at-death proxy across different populations102,103,104.
Digitization and morphological analysis
Most Iberian specimens were CT scanned using a Toshiba Astelion scanner with an algorithm optimized for scanning bone (120 kV, voxel size 0.348 × 0.348 × 0.3, revolution time 0.75 s, spiral pitch factor 0.94) at the Faculty of Veterinary Medicine of the University of Lisbon. A smaller number of the Iberian specimens was digitized using an Einscan Pro 2X Plus surface scanner. Levantine specimens were digitized using the same scanning algorithm used for scanning the Iberian specimens (120 kV., voxel size 0.307 × 0.307 × 0.6 mm., revolution time 0.75 s, spiral pitch factor 0.94) at the Koret School of Veterinary Medicine (Rehovot, Israel).
In the case of the CT-scanned mandibles, segmentation was performed in 3D Slicer105 using standard protocols106,107,108,109. Specimens that were fragmented were pieced together virtually110. Extraction of landmark (LM) coordinates ensued using a set of 21 anatomical LMs (Table 4). In incomplete specimens, the function estimate.missing of the R package geomorph111 was used to estimate the original location of the missing LMs geometrically (i.e., Thin Plate Splines rather than multivariate regression based reconstruction was used). To that end, population specific reference mean specimens were used to reconstruct incomplete specimens (i.e., the mean of the complete Iberian Mesolithic mandibles was used as reference to estimate the missing regions of the incomplete Iberian Mesolithic mandibles; similarly, Neolithic Iberian specimens were used as reference to reconstruct incomplete specimens from that group), although a previous sensitivity study showed negligible to no differences in the outcome of population specific and non-population specific reconstruction of modern human mandibles46. The former reconstruction approach was chosen because previous studies also show that using inadequate references (i.e., references from populations that are morphologically meaningfully different from the target reconstructed specimens) may lead to large estimation errors112,113,114. Even though 66 hemi-mandibles were partially reconstructed using this approach (Table SI 3), many other specimens were excluded because reconstruction of excessively incomplete mandibles (i.e., with more than ~ 5 missing LMs when using the LM set of this study) may result in meaningful reconstruction error and so in biased results115.
Standard GM analyses using the full sample followed reconstruction of the incomplete specimens in the R geomorph package111 and EVAN toolbox116,117. First, Generalized Procrustes Analysis (GPA) was used to superimpose all landmark configurations and remove the effects of location, size and orientation on the raw coordinates118,119. The resulting shape variables were then used to examine morphological variance and hypothetical similarities and/or differences between groups. To that end, Principal Component Analysis (PCA) was used to reduce dimensionality and examine shape differences between specimens, which were visualized warping a surface along the relevant PCs. Size differences between specimens and groups were assessed using centroid size.
Specimens were grouped based on chronology and region (i.e., Mesolithic Iberia, Levantine Chalcolithic, etc.). Potential differences in size (i.e., centroid size) were examined using the Kruskal–Wallis test. Shape differences were first tested using a nonparametric test Permutational Multivariate ANOVA (PERMANOVA) to assess potential multivariate shape differences in the different groups120. This was based on the first 29 PCs, which explain ~ 95% of the total variance and was implemented in Past121 using 10,000 permutations. The Kruskal–Wallis test (followed by post-hoc tests) was used to test for differences in the two first PCs, which were used together with surface warping to visualize shape differences across the groups. Kruskal–Wallis tests were implemented using the R package ggstatsplot122. The use of non-parametric testing was necessary for centroid size and PCs 1 and 2 after the Shapiro–Wilk's test revealed that the ANOVA assumption of normality of residuals was not met for size, and Levene's test of homogeneity of variances was not met for shape. These tests, along with the Durbin Watson test for independence of residuals, were carried out in the car R package123. Lastly, the morphol.disparity function of the geomorph package111 was used to examine hypothetical differences in shape variance across groups (i.e., disparity using Procrustes distances).
Relationship between dental wear magnitude and mandibular morphology
Because dental wear relates to diet and masticatory function, we used it as a proxy to examine differences in the material properties of foodstuffs eaten by the different groups examined. To that end, dental wear magnitude was scored for each tooth according to the eight stage ordinal scale of B.H. Smith 47. Previous studies (based on part of the sample used in this present study), have shown that ante and postmortem tooth loss had resulted in the absence of many teeth25. This is especially pervasive in the anterior dentition, in which only 28/101 (27.72%) of lower central right incisors were present, compared to 75/101 (74.26%) of lower left first molars (Table SI 1). To mitigate this effect, antimeres were pooled, and wear scores averaged. The wear score of single teeth was used whenever antimeres were absent. As in other studies using this approach25,103,124, this procedure ensued after we found no statistically significant differences in the magnitude of wear between antimeres, using Wilcoxon pairwise comparisons in R.
Even though dental wear was used in this study as a proxy for differences in diet and masticatory function, it is also influenced by age54,100,101. Regrettably, the nature of the samples did not allow age-at-death estimation based on skeletal elements other than the mandible. Thus, in addition to quantifying dental wear of individual teeth, we also compared rates of dental wear of paired first and second molars. This approach was used because it has been demonstrated to be age independent and used in other studies to compare the rate at which teeth are worn, which should relate to the material properties of foodstuffs processed intra-orally54,99,125.
Dental wear was scored by the same observer (RMG), preventing inter-observer error. Although not all teeth were observed multiple times, a previous study with a sub-sample (n = 412) of the one used in this study showed no scoring differences between repeated observations by the same observer126. Dental wear scores of paired first and second molars were plotted using the ggplot2 package of R92. Statistical testing of dental wear magnitude differences between groups were carried out using the non-parametric Kruskal–Wallis test, together with post-hoc tests, which were implemented using the ggstatsplot R package122. Non-parametric testing was necessary because ANOVA assumptions (i.e., homogeneity of variance, normal distribution and independence of residuals) were not met.
To explore the relationship between dental wear and mandibular morphology, multiple regression was used to regress the scores of the first 29 PCs against wear magnitude scores of the first and second molars (which yielded the largest samples and statistically significant differences between groups) in R. The regression analysis was undertaken independently for each group.
Data availability
The data that support this study is available from the corresponding author upon reasonable request.
References
Barker, G. & Goucher, C. The Cambridge World History: Volume 2: A World with Agriculture, 12,000 BCE–500 CE. Vol. 2 (Cambridge University Press, 2015).
Fernández, E. et al. Ancient DNA analysis of 8000 B.C. near eastern farmers supports an early neolithic pioneer maritime colonization of Mainland Europe through Cyprus and the Aegean Islands. PLOS Genet. 10, e1004401. https://doi.org/10.1371/journal.pgen.1004401 (2014).
Mathieson, I. et al. Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503. https://doi.org/10.1038/nature16152 (2015).
Lazaridis, I. et al. Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424. https://doi.org/10.1038/nature19310 (2016).
Haber, M. et al. Continuity and admixture in the last five millennia of Levantine history from ancient Canaanite and present-day Lebanese genome sequences. Am. J. Hum. Genet. 101, 274–282. https://doi.org/10.1016/j.ajhg.2017.06.013 (2017).
Feldman, M. et al. Late Pleistocene human genome suggests a local origin for the first farmers of central Anatolia. Nat. Commun. 10, 1218. https://doi.org/10.1038/s41467-019-09209-7 (2019).
Hofmanová, Z. et al. Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. 113, 6886–6891. https://doi.org/10.1073/pnas.1523951113 (2016).
Omrak, A. et al. Genomic evidence establishes Anatolia as the source of the European Neolithic gene pool. Curr. Biol. 26, 270–275. https://doi.org/10.1016/j.cub.2015.12.019 (2016).
Martins, H. et al. Radiocarbon dating the beginning of the Neolithic in Iberia: New results, new problems. J. Mediterr. Archaeol. 28, 105–131 (2015).
Zilhão, J. Europe’s First Farmers 144–182 (Cambridge University Press, 2000).
Zilhão, J. Radiocarbon evidence for maritime pioneer colonization at the origins of farming in west Mediterranean Europe. Proc. Natl. Acad. Sci. 98, 14180–14185. https://doi.org/10.1073/pnas.241522898 (2001).
Villalba-Mouco, V. et al. Survival of Late Pleistocene hunter-gatherer ancestry in the Iberian Peninsula. Curr. Biol. 29, 1169-1177.e1167. https://doi.org/10.1016/j.cub.2019.02.006 (2019).
Olalde, I. et al. A common genetic origin for early farmers from Mediterranean Cardial and Central European LBK cultures. Mol. Biol. Evol. 32, 3132–3142. https://doi.org/10.1093/molbev/msv181 (2015).
Haak, W. et al. Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211. https://doi.org/10.1038/nature14317 (2015).
Olalde, I. et al. The genomic history of the Iberian Peninsula over the past 8000 years. Science 363, 1230. https://doi.org/10.1126/science.aav4040 (2019).
Carvalho, A. F. et al. Hunter-gatherer genetic persistence at the onset of megalithism in western Iberia: New mitochondrial evidence from Mesolithic and Neolithic necropolises in central-southern Portugal. Quat. Int. https://doi.org/10.1016/j.quaint.2023.03.015 (2023).
Pinhasi, R. & Stock, J. T. Human Bioarchaeology of The Transition to Agriculture. (Wiley-Blackwell, Chichester, 2011).
Larsen, C. S. Bioarchaeology: Interpreting Behavior from the Human Skeleton (Cambridge University Press, 1997).
Katz, D. C., Grote, M. N. & Weaver, T. D. Changes in human skull morphology across the agricultural transition are consistent with softer diets in preindustrial farming groups. Proc. Natl. Acad. Sci. https://doi.org/10.1073/pnas.1702586114 (2017).
von Cramon-Taubadel, N. Global human mandibular variation reflects differences in agricultural and hunter-gatherer subsistence strategies. Proc. Natl. Acad. Sci. 108, 19546–19551. https://doi.org/10.1073/pnas.1113050108 (2011).
Pokhojaev, A., Avni, H., Sella-Tunis, T., Sarig, R. & May, H. Changes in human mandibular shape during the Terminal Pleistocene-Holocene Levant. Sci. Rep. 9, 8799. https://doi.org/10.1038/s41598-019-45279-9 (2019).
May, H., Sella-Tunis, T., Pokhojaev, A., Peled, N. & Sarig, R. Changes in mandible characteristics during the terminal Pleistocene to Holocene Levant and their association with dietary habits. J. Archaeol. Sci.: Rep. 22, 413–419. https://doi.org/10.1016/j.jasrep.2018.03.020 (2018).
Galland, M., Van Gerven, D. P., Von Cramon-Taubadel, N. & Pinhasi, R. 11,000 years of craniofacial and mandibular variation in Lower Nubia. Sci. Rep. 6, 31040. https://doi.org/10.1038/srep31040 (2016).
Lubell, D., Jackes, M., Schwarcz, H., Knyf, M. & Meiklejohn, C. The Mesolithic–Neolithic transition in Portugal: Isotopic and dental evidence of diet. J. Archaeol. Sci. 21, 201–216 (1994).
Godinho, R. M., Umbelino, C., Garcia, S. & Gonçalves, C. Changes in dental wear magnitude in the last ∼8000 years in southwestern Iberia. Arch. Oral Biol. 147, 105626. https://doi.org/10.1016/j.archoralbio.2023.105626 (2023).
Schmidt, C. W. et al. Dental microwear texture analysis of Homo sapiens sapiens: Foragers, farmers, and pastoralists. Am. J. Phys. Anthropol. 169, 207–226. https://doi.org/10.1002/ajpa.23815 (2019).
Calandra, I. & Merceron, G. Dental microwear texture analysis in mammalian ecology. Mamm. Rev. 46, 215–228. https://doi.org/10.1111/mam.12063 (2016).
Hua, L.-C., Brandt, E. T., Meullenet, J.-F., Zhou, Z.-R. & Ungar, P. S. Technical note: An in vitro study of dental microwear formation using the BITE Master II chewing machine. Am. J. Phys. Anthropol. 158, 769–775. https://doi.org/10.1002/ajpa.22823 (2015).
Guiry, E. J. et al. The transition to agriculture in south-western Europe: New isotopic insights from Portugal’s Atlantic coast. Antiquity 90, 604–616. https://doi.org/10.15184/aqy.2016.34 (2016).
Cubas, M. et al. Long-term dietary change in Atlantic and Mediterranean Iberia with the introduction of agriculture: A stable isotope perspective. Archaeol. Anthropol. Sci. 11, 3825–3836. https://doi.org/10.1007/s12520-018-0752-1 (2019).
Carvalho, A. F. et al. The Bom Santo Cave (Lisbon, Portugal): Catchment, diet, and patterns of mobility of a Middle Neolithic Population. Eur. J. Archaeol. 19, 187–214. https://doi.org/10.1179/1461957115y.0000000014 (2016).
Umbelino, C. Outros sabores do passado: as análises de oligoelementos e de isótopos estáveis na reconstituição da dieta das comunidades humanas do Mesolítico Final e do Neolítico Final-Calcolítico do território português, University of Coimbra (2006).
Bicho, N. et al. Resilience, replacement and acculturation in the Mesolithic/Neolithic transition: The case of Muge, central Portugal. Quat. Int. 446, 31–42. https://doi.org/10.1016/j.quaint.2016.09.049 (2017).
Quagliariello, A. et al. Ancient oral microbiomes support gradual Neolithic dietary shifts towards agriculture. Nat. Commun. 13, 6927. https://doi.org/10.1038/s41467-022-34416-0 (2022).
Bocquentin, F., Chamel, B., Anton, M. & Noûs, C. The subsistence and foodways transition during the Neolithization process. Glimpses from a contextualized dental perspective. Food Hist. 19, 23–52 (2021).
Smith, P. & Horwitz, L. K. Ancestors and inheritors: A bio-cultural perspective of the transition to agropastoralism in the Southern Levant. In Ancient Health. Skeletal Indicators of Agricultural and Economic Intensification (eds Cohen, M. N. & Crane-Kramer, G. M. M.) 207–222 (University Press of Florida, Florida, 2007).
Douglas, M. T. & Pietrusewsky, M. in Ancient Health: Skeletal Indicators of Agricultural Economic Intensification (eds Cohen, M. N. & Crane-Kramer, G. M. M.) 300–319 (University Press of Florida, 2007).
Halcrow, S. E., Harris, N. J., Tayles, N., Ikehara-Quebral, R. & Pietrusewsky, M. From the mouths of babes: Dental caries in infants and children and the intensification of agriculture in mainland Southeast Asia. Am. J. Phys. Anthropol. 150, 409–420. https://doi.org/10.1002/ajpa.22215 (2013).
Cohen, M. N. & Armelagos, G. J. Paleopathology at the Origins of Agriculture (Academic Press, 1984).
Cohen, M. N. & Crane-Kramer, G. M. M. Ancient Health: Skeletal Indicators of Agricultural and Economic Intensification. (University Press of Florida, Florida, 2007).
von Cramon-Taubadel, N. & Pinhasi, R. Craniometric data support a mosaic model of demic and cultural Neolithic diffusion to outlying regions of Europe. Proc. R. Soc. London B: Biol. Sci. https://doi.org/10.1098/rspb.2010.2678 (2011).
Pinhasi, R. & von Cramon-Taubadel, N. Craniometric data supports demic diffusion model for the spread of agriculture into Europe. PLoS ONE 4, e6747 (2009).
Jackes, M., Lubell, D. & Meiklejohn, C. On physical anthropological aspects of the Mesolithic–Neolithic transition in the Iberian Peninsula. Curr. Anthropol. 38, 839–846. https://doi.org/10.1086/204670 (1997).
Buck, T. J. & Vidarsdottir, U. S. A proposed method for the identification of race in sub-adult skeletons: A geometric morphometric analysis of mandibular morphology. J. Forensic Sci. 49, JFS2004074-2004076 (2004).
Mounier, A. et al. Who were the Nataruk people? Mandibular morphology among late Pleistocene and early Holocene fisher-forager populations of West Turkana (Kenya). J. Hum. Evol. 121, 235–253. https://doi.org/10.1016/j.jhevol.2018.04.013 (2018).
Godinho, R. M., Umbelino, C. & Gonçalves, C. Mesolithic and Chalcolithic mandibular morphology: Using geometric morphometrics to reconstruct incomplete specimens and analyse morphology. Open Archaeol. 8, 536–549. https://doi.org/10.1515/opar-2022-0247 (2022).
Smith, B. H. Patterns of molar wear in hunter–gatherers and agriculturalists. Am. J. Phys. Anthropol. 63, 39–56. https://doi.org/10.1002/ajpa.1330630107 (1984).
Lucas, P. W. Dental Functional Morphology—How Teeth Work (Cambridge University Press, 2004).
Molnar, S. et al. Tooth wear and culture: A survey of tooth functions among some prehistoric populations [and comments and reply]. Curr. Anthropol. 13, 511–526. https://doi.org/10.1086/201284 (1972).
Deter, C. A. Gradients of occlusal wear in hunter-gatherers and agriculturalists. Am. J. Phys. Anthropol. 138, 247–254. https://doi.org/10.1002/ajpa.20922 (2009).
Bernal, V., Novellino, P., Gonzalez, P. N. & Perez, S. I. Role of wild plant foods among late Holocene hunter-gatherers from Central and North Patagonia (South America): An approach from dental evidence. Am. J. Phys. Anthropol. 133, 1047–1059. https://doi.org/10.1002/ajpa.20638 (2007).
Eshed, V., Gopher, A. & Hershkovitz, I. Tooth wear and dental pathology at the advent of agriculture: New evidence from the Levant. Am. J. Phys. Anthropol. 130, 145–159. https://doi.org/10.1002/ajpa.20362 (2006).
Kaifu, Y. Changes in the pattern of tooth wear from prehistoric to recent periods in Japan. Am. J. Phys. Anthropol. 109, 485–499 (1999).
Smith, P. Diet and attrition in the natufians. Am. J. Phys. Anthropol. 37, 233–238. https://doi.org/10.1002/ajpa.1330370207 (1972).
Currey, J. D. Bones, Structure and Mechanics (Princeton University Press, 2006).
Judex, S. & Rubin, C. T. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity?. J. Musculoskelet. Neuronal Interact. 10, 3–11 (2010).
Judex, S., Gross, T. S. & Zernicke, R. F. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J. Bone Miner. Res. 12, 1737–1745. https://doi.org/10.1359/jbmr.1997.12.10.1737 (1997).
Judex, S., Lei, X., Han, D. & Rubin, C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J. Biomech. 40, 1333–1339. https://doi.org/10.1016/j.jbiomech.2006.05.014 (2007).
Lanyon, L. E. & Rubin, C. T. Static Vs dynamic loads as an influence on bone remodeling. J. Biomech. 17, 897–905. https://doi.org/10.1016/0021-9290(84)90003-4 (1984).
Lanyon, L. E. Functional strain as a determinant for bone remodeling. Calcif. Tissue Int. 36, S56–S61. https://doi.org/10.1007/bf02406134 (1984).
Turner, C. H. Three rules for bone adaptation to mechanical stimuli. Bone 23, 399–407. https://doi.org/10.1016/S8756-3282(98)00118-5 (1998).
Cunha, C. Crossing the river: The dental morphology of Chalcolithic populations in the Middle Guadiana PhD thesis, University of Coimbra, (2015).
Godinho, R. M., Santos, A. L. & Valera, A. C. A lunate-triquetral coalition from a commingled funerary context from the Chalcolithic Perdigões ditched enclosures of Portugal. Anthropol. Anz. 77, 83–88. https://doi.org/10.1127/anthranz/2019/0935 (2020).
Lillios, K. T. in The Archaeology of the Iberian Peninsula: From the Paleolithic to the Bronze Age Cambridge World Archaeology (ed. Lillios, K. T.) 171–226 (Cambridge University Press, 2019).
Cardoso, J. L. Copper metallurgy and the importance of other raw materials in the context of Chalcolithic economic intensification in Portuguese Estremadura. J. Iberian Archaeol. 1, 94–109 (1999).
Schuhmacher, T. X., Cardoso, J. L. & Banerjee, A. Sourcing African ivory in Chalcolithic Portugal. Antiquity 83, 983–997. https://doi.org/10.1017/S0003598X00099294 (2009).
Schuhmacher, T. X. in Key resources and sociocultural developments in the Iberian chalcolithic. 291–312 (Tübingen Library Publishing, 2017).
Valera, A. C., Schuhmacher, T. X. & Banerjee, A. Ivory in the Chalcolithic enclosure of Perdigões (South Portugal): The social role of an exotic raw material. World Archaeol. 47, 390–413. https://doi.org/10.1080/00438243.2015.1014571 (2015).
Valera, A. C. in Key Resources and Sociocultural Developments in the Iberian Chalcolithic. (eds Bartelheim, M., Ramírez, P. B. & Kunst, M.) 201–224 (Tübingen Library Publishing, 2017).
Bonilla, M. D. Z., Beck, J., Bocherens, H. & Díaz-del-Río, P. Isotopic evidence for mobility at large-scale human aggregations in Copper Age Iberia: The mega-site of Marroquíes. Antiquity 92, 991–1007. https://doi.org/10.15184/aqy.2018.33 (2018).
Valera, A. C. et al. Addressing human mobility in Iberian Neolithic and Chalcolithic ditched enclosures: The case of Perdigões (South Portugal). J. Archaeol. Sci.: Rep. 30, 102264. https://doi.org/10.1016/j.jasrep.2020.102264 (2020).
Waterman, A. J., Peate, D. W., Silva, A. M. & Thomas, J. T. In search of homelands: using strontium isotopes to identify biological markers of mobility in late prehistoric Portugal. J. Archaeol. Sci. 42, 119–127. https://doi.org/10.1016/j.jas.2013.11.004 (2014).
Ruff, C. B. et al. Gradual decline in mobility with the adoption of food production in Europe. Proc. Natl. Acad. Sci. 112, 7147 (2015).
Holt, B., Whittey, E., Niskanen, M., Sládek, V., Berner, M. & Ruff, C. B. in Skeletal Variation and Adaptation in Europeans: Upper Paleolithic to the Twentieth Century (ed. Ruff, C. B.) 91–132 (John Wiley & Sons, Inc., 2018).
Ruff, C. B. et al. Body size, body proportions, and mobility in the Tyrolean “Iceman”. J. Human Evol. 51, 91–101. https://doi.org/10.1016/j.jhevol.2006.02.001 (2006).
Marchi, D., Sparacello, V. & Shaw, C. in Human Bioarchaeology of the Transition to Agriculture 317–346 (2011).
Ruff, C. B. & Garvin, H. in Skeletal Variation and Adaptation in Europeans 281–314 (2018).
Harney, É. et al. Ancient DNA from Chalcolithic Israel reveals the role of population mixture in cultural transformation. Nat. Commun. 9, 3336. https://doi.org/10.1038/s41467-018-05649-9 (2018).
Lipson, M. et al. Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature 551, 368–372. https://doi.org/10.1038/nature24476 (2017).
Bastir, M. & Rosas, A. Comparative ontogeny in humans and chimpanzees: Similarities, differences and paradoxes in postnatal growth and development of the skull. Ann. Anat.- Anatomischer Anzeiger 186, 503–509. https://doi.org/10.1016/S0940-9602(04)80096-7 (2004).
Singh, N. Ontogenetic study of allometric variation in Homo and Pan mandibles. Anat. Rec. 297, 261–272. https://doi.org/10.1002/ar.22843 (2014).
Coquerelle, M. et al. Sexual dimorphism of the human mandible and its association with dental development. Am. J. Phys. Anthropol. 145, 192–202. https://doi.org/10.1002/Ajpa.21485 (2011).
Rosas, A. & Bastir, M. Thin-plate spline analysis of allometry and sexual dimorphism in the human craniofacial complex. Am. J. Phys. Anthropol. 117, 236–245. https://doi.org/10.1002/ajpa.10023 (2002).
Alarcón, J. A., Bastir, M. & Rosas, A. Variation of mandibular sexual dimorphism across human facial patterns. HOMO – J. Comp. Hum. Biol. 67, 188–202. https://doi.org/10.1016/j.jchb.2015.11.004 (2016).
Tunis, T. S. et al. Sex estimation using computed tomography of the mandible. Int. J. Legal Med. 131, 1691–1700. https://doi.org/10.1007/s00414-017-1554-1 (2017).
Franklin, D., O’Higgins, P. & Oxnard, C. E. Sexual dimorphism in the mandible of indigenous South Africans: A geometric morphometric approach. S. Afr. J. Sci. 104, 101–106 (2008).
Buikstra, J. & Ubelaker, D. Standards for Data Collection from Human Skeletal Remains: Proceedings of a Seminar at the Field Museum of Natural History. (Arkansas Archeological Survey, 1994).
Ferembach, D., Schwidetzky, I. & Stloukal, M. Recommendations for age and sex diagnoses of skeletons. J. Hum. Evol. 9, 517–549. https://doi.org/10.1016/0047-2484(80)90061-5 (1980).
Kimmerle, E. H., Ross, A. & Slice, D. Sexual dimorphism in America: Geometric morphometric analysis of the craniofacial region. J. Forensic Sci. 53, 54–57. https://doi.org/10.1111/j.1556-4029.2007.00627.x (2008).
Kleisner, K. et al. How and why patterns of sexual dimorphism in human faces vary across the world. Sci. Rep. 11, 5978. https://doi.org/10.1038/s41598-021-85402-3 (2021).
Package ‘rnaturalearth’: World Map Data from Natural Earth. R package version 0.3.4. 1–16 v. 0.3.4 (2023).
Wickham, H. et al. ggplot2: Create elegant data visualisations using the grammar of graphics. R package version 3.3.4. 1–189 (2021).
AlQahtani, S. J., Hector, M. P. & Liversidge, H. M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 142, 481–490. https://doi.org/10.1002/ajpa.21258 (2010).
Jackes, M. & Meiklejohn, C. Building a method for the study of the Mesolithic–Neolithic transition in Portugal. Documenta Praehistorica 31, 89–111 (2004).
Valera, A. C., Santos, H., Figueiredo, M. & Granja, R. in 4° Colóquio de Arqueologia do Alqueva (eds António Carlos Silva, Frederico Tátá Regala, & Miguel Martinho) 83–95 (EDIA/DRCAlen, 2014).
Miguel, L. & Simão, P. Minimização de Impactes sobre o Património Cultural decorrentes da execução dos Blocos de Rega de Pias (Fase de Obra) - Relatório dos Trabalhos Arqueológicos Monte da Guarita 2 (2017). (Era Arqueologia, S.A., Lisboa, 2017).
Antunes-Ferreira, N. Paleobiologia de grupos populacionais do Neolítico final/Calcolítico do Poço Velho (Cascais). (Instituto português de arqueologia, 2005).
Carvalho, A., Gonçalves, D., Granja, R. & Petchey, F. in Funerary Practices in the Iberian Peninsula From the Mesolithic to The Chalcolithic (eds Gibaja, J. F., Carvalho, A. & Chambon, P.) (2012).
Chattah, N.L.-T. & Smith, P. Variation in occlusal dental wear of two Chalcolithic populations in the southern Levant. Am. J. Phys. Anthropol. 130, 471–479. https://doi.org/10.1002/ajpa.20388 (2006).
Miles, A. E. W. The miles method of assessing age from tooth wear revisited. J. Archaeol. Sci. 28, 973–982. https://doi.org/10.1006/jasc.2000.0652 (2001).
Scott, E. C. Principal axis analysis of dental attrition data. Am. J. Phys. Anthropol. 51, 203–211. https://doi.org/10.1002/ajpa.1330510207 (1979).
Benazzi, S., Bonetti, C., Cilli, E. & Gruppioni, G. Molar crown height: not always a reliable method for the evaluation of age-at-death. J. Archaeol. Sci. 35, 2371–2378. https://doi.org/10.1016/j.jas.2008.03.005 (2008).
Clement, A. F. & Hillson, S. W. Intrapopulation variation in macro tooth wear patterns—a case study from Igloolik, Canada. Am. J. Phys. Anthropol. 149, 517–524. https://doi.org/10.1002/ajpa.22153 (2012).
Romero, A., Ramirez-Rozzi, F. V., Cuesta-Torralvo, E. & Pérez-Pérez, A. Age-related tooth wear in African rainforest hunter-gatherers. Am. J. Phys. Anthropol. 170, 622–628. https://doi.org/10.1002/ajpa.23934 (2019).
Fedorov, A. et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Resonance Imaging 30, 1323–1341. https://doi.org/10.1016/j.mri.2012.05.001 (2012).
Godinho, R. M. & O'Higgins, P. in Human remains - Another Dimension: The Application of 3D Imaging in Funerary Context (eds Errickson, D. & Thompson, T.) 135–147 (Elsevier, 2017).
Godinho, R. M. & O’Higgins, P. The biomechanical significance of the frontal sinus in Kabwe 1 (Homo heidelbergensis). J. Hum. Evol. 114, 141–153. https://doi.org/10.1016/j.jhevol.2017.10.007 (2018).
Godinho, R. M., Spikins, P. & O’Higgins, P. Supraorbital morphology and social dynamics in human evolution. Nat. Ecol. Evol. 2, 956–961. https://doi.org/10.1038/s41559-018-0528-0 (2018).
Godinho, R. M. et al. The biting performance of Homo sapiens and Homo heidelbergensis. J. Hum. Evol. 118, 56–71. https://doi.org/10.1016/j.jhevol.2018.02.010 (2018).
Godinho, R. M. & Gonçalves, C. in Actas do III Congresso da Associação dos Arqueólogos Portugueses (eds Arnaud, J. M., Neves, C. & Martins, A.) 311–323 (Associação dos Arqueólogos Portugueses e CITCEM, 2020).
Geomorph: Software for geometric morphometric analyses. R package version 3.3.2 (2021).
Gunz, P., Mitteroecker, P., Neubauer, S., Weber, G. W. & Bookstein, F. L. Principles for the virtual reconstruction of hominin crania. J. Hum. Evol. 57, 48–62. https://doi.org/10.1016/j.jhevol.2009.04.004 (2009).
Neeser, R., Ackermann, R. R. & Gain, J. Comparing the accuracy and precision of three techniques used for estimating missing landmarks when reconstructing fossil hominin crania. Am. J. Phys. Anthropol. 140, 1–18. https://doi.org/10.1002/ajpa.21023 (2009).
Senck, S., Bookstein, F. L., Benazzi, S., Kastner, J. & Weber, G. W. Virtual reconstruction of modern and fossil hominoid crania: Consequences of reference sample choice. Anat. Rec. 298, 827–841. https://doi.org/10.1002/ar.23104 (2015).
Godinho, R. M., O’Higgins, P. & Gonçalves, C. Assessing the reliability of virtual reconstruction of mandibles. Am. J. Phys. Anthropol. 172, 723–734. https://doi.org/10.1002/ajpa.24095 (2020).
O'Higgins, P., Weber, G., Baverstock, H., Proa, M., Dunn, J. & Fornai, C. Manuals for the EVAN Toolbox: No.1 Templand. (2012).
Weber, G. & Bookstein, F. Manuals for the EVAN Toolbox: No.2. (2012).
Zelditch, M. L., Swiderski, D. L., Sheets, H. D. & Fink, W. L. Geometric Morphometrics for Biologists: A Primer (Elsevier, 2004).
O’Higgins, P. The study of morphological variation in the hominid fossil record: Biology, landmarks and geometry. J. Anat. 197, 103–120. https://doi.org/10.1046/j.1469-7580.2000.19710103.x (2000).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x (2001).
Hammer, O. PAST reference manual 4.12. (2022).
Patil, I. Visualizations with statistical details: The’ggstatsplot’approach. J. Open Source Softw. 6, 3167 (2021).
Fox, J. et al. car: Companion to Applied Regression. (2012).
Molnar, S., Richards, L., McKee, J. & Molnar, I. Tooth wear in Australian Aboriginal populations from the river Murray valley. Am. J. Phys. Anthropol. 79, 185–196. https://doi.org/10.1002/ajpa.1330790206 (1989).
Watson, J. T. Changes in food processing and occlusal dental wear during the early agricultural period in northwest Mexico. Am. J. Phys. Anthropol. 135, 92–99. https://doi.org/10.1002/ajpa.20712 (2008).
Godinho, R. M. & Gonçalves, C. Testing the reliability of CT scan-based dental wear magnitude scoring. Am. J. Phys. Anthropol. 176, 521–527. https://doi.org/10.1002/ajpa.24374 (2021).
Acknowledgements
RM Godinho is funded by Fundação para a Ciência e a Tecnologia (FCT; contract reference 2020.00499.CEECIND). This research was also funded by: FCT R&D projects (project “ParaFunction—Are Neanderthals adapted to heavy masticatory and paramasticatory function?”, reference 2022.07737.PTDC; https://doi.org/10.54499/2022.07737.PTDC); the Synthesys+ program (project reference IL-TAF-98, The Hebrew University of Jerusalem). Thanks are also due to the FCT for funding of the following projects: the European Regional Development Fund (FEDER) via the Programa Operacional CRESC Algarve 2020, of Portugal2020, in the context of the MugePortal project (“Muge Shellmiddens Project: a new portal for the last hunter-gatherers of the Tagus Valley, Portugal (MugePortal)”, project reference ALG-01-0145-FEDER-29680/ PTDC/HAR-ARQ/29680/2017); “Os últimos caçadores‑recolectores de Muge (Portugal): as origens da complexidade social” (PTDC/HIS‑ ARQ/112156/2009); “Os últimos caçadores‑ recolectores do vale do Tejo—os concheiros de Muge” (PTDC/HAH/64185/2006). The EarthWatch Institute funded “Discovering Ancient Societies in Portugal”. C. Umbelino is funded by the Fundação para a Ciência e Tecnologia (FCT, reference UIDB/00283/2020). J Cascalheira is funded by the Portuguese Foundation for Science and Technology (FCT; contract reference DL 57/2016/CP1361/CT0026). C Gonçalves is funded by the Portuguese Foundation for Science and Technology (FCT; contract reference DL 57/2016/CP1361/CT0029). This research was also funded by the Archeological Institute of America (The Archeology of Portugal Fellowship). Thanks are due to Dr Miguel Ramalho† and Dr. José António Moita for granting access to the samples housed at Museu Geológico e Mineiro; Dr. António Carvalho, Dr. Luísa Guerreiro and Dr. Paulo Alves for access to the specimens housed at the Museu Nacional de Arqueologia; Dr. Rita Gaspar and the Museu de História Natural da Universidade do Porto for access to the specimens housed therein; Era Arqueologia S.A. for providing access to the skeletal remains from Perdigões and Monte da Guarita 2; Sara Ramos and Museu Municipal de Ferreira do Alentejo for access to the samples from Monte do Carrascal 2; Prof. Sandra Jesus and Dr. Óscar Gamboa for CT scanning at the Faculty of Veterinary Medicine of the University of Lisbon. The specimens from the Levant studied here are curated in the National Natural History Collections at the Hebrew University. Thanks to Prof. Gila Kahila Bar-Gal and Prof. Itzhak (Zahi) Aizenberg for CT scanning at the Bet Dagon Veterinary Hospital, Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, and Dr. Gadi Herzlinger and Prof. Leore Grosman for surface scanning of these latter specimens. Thanks to Godefroy Devevey for discussions on specific aspects of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed for this research. Conceptualization: R.M.G., P.S., C.G.; Methodology: R.M.G., P.S.; Data collection: R.M.G.; Data analysis: R.M.G.; C.U., P.S.; Drafting; R.M.G., C.U., A.C.V., A.F.C., N.B., J.C., C.G., P.S.; Final approval of the submitted version: R.M.G., C.U., A.C.V., A.F.C., N.B., J.C., C.G., P.S.; Funding acquisition: R.M.G., P.S., C.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Godinho, R.M., Umbelino, C., Valera, A.C. et al. Mandibular morphology and the Mesolithic–Neolithic transition in Westernmost Iberia. Sci Rep 13, 16648 (2023). https://doi.org/10.1038/s41598-023-42846-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42846-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.