Abstract
Glioblastoma, a malignant tumor, has no curative treatment. Recently, mitochondria have been considered a potential target for treating glioblastoma. Previously, we reported that agents initiating mitochondrial dysfunction were effective under glucose-starved conditions. Therefore, this study aimed to develop a mitochondria-targeted treatment to achieve normal glucose conditions. This study used U87MG (U87), U373, and patient-derived stem-like cells as well as chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG). We investigated whether CAP and 2-DG inhibited the growth of cells under normal and high glucose concentrations. In U87 cells, 2-DG and long-term CAP administration were more effective under normal glucose than high-glucose conditions. In addition, combined CAP and 2-DG treatment was significantly effective under normal glucose concentration in both normal oxygen and hypoxic conditions; this was validated in U373 and patient-derived stem-like cells. 2-DG and CAP acted by influencing iron dynamics; however, deferoxamine inhibited the efficacy of these agents. Thus, ferroptosis could be the underlying mechanism through which 2-DG and CAP act. In conclusion, combined treatment of CAP and 2-DG drastically inhibits cell growth of glioblastoma cell lines even under normal glucose conditions; therefore, this treatment could be effective for glioblastoma patients.
Similar content being viewed by others
Introduction
Glioblastoma is one of the most malignant brain tumors with a poor prognosis and no curative treatment1,2,3. Temozolomide is a standard treatment for glioblastoma; however, it prolongs survival by only 2.5 months3. Furthermore, glioblastoma cells become resistant to temozolomide, and the mitochondria are associated with this resistance3,4,5.
The mitochondria are important in adenosine triphosphate production, calcium dynamics, β-oxidation, and reactive oxygen species generation6. Mitochondria are also associated with various diseases, including malignant tumors, especially in cancer stem cells7,8. Thus, we investigated a mitochondria-targeted treatment for glioblastoma.
Our previous study found that antimicrobial agents, including chloramphenicol (CAP) and doxycycline, are effective for treating glioblastoma under glucose-starved conditions because glioblastoma cells become dependent on the mitochondria in this condition9. A 3-day administration of these antimicrobial agents was ineffective in normal or high glucose concentrations9. Consequently, in this study, we aimed to develop an effective treatment for glioblastoma in conditions of normal glucose concentration.
2-Deoxy-d-glucose (2-DG), a glucose analog, inhibits hexokinase and phosphoglucose isomerase activities, blocking glycolysis10. Moreover, 2-DG mimics glucose-starved conditions, induces mitochondrial oxidative stress, and inhibits cancer cell growth10. Although Singh et al.11 reported combined treatment with 2-DG and radiotherapy for glioblastoma treatment, this approach is not used in clinical practice. However, 2-DG was used in some clinical trials for cancers or solid tumors12,13.
In the tumor environment, cells exist in glucose-starved and hypoxic conditions, which should be considered in the development of treatments for glioblastoma14,15. In this study, treatment efficacy was investigated in both normal and hypoxic conditions. In addition, results from cell line analysis were validated using stem-like cells mimicking an in vivo condition.
The mechanisms underlying cell death caused by each agent vary, including apoptosis, necroptosis, and ferroptosis9,16. Ferroptosis is a form of cell death that is currently attracting attention. In our previous study, CAP resulted in cell death via ferroptosis under glucose-starved conditions9. Ferroptosis has various markers, including FTH1, GpX4, KEAP1, and NRF2. Additionally, mRNA, PTGS2, CHAC1, and HO-1 are related to ferroptosis17. One of the inhibitors of the ferroptosis pathway is deferoxamine (DFO)9.
This study aimed to investigate the efficacy of a mitochondria-targeted treatment under a normal glucose condition. We examined the efficacy of CAP and 2-DG individually and combined. In addition, we investigated the mechanisms underlying cell death caused by these agents.
Results
Chloramphenicol (CAP) with glucose control is effective in the long term
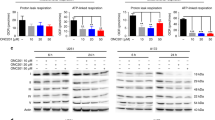
In our previous study, CAP was only effective under glucose-starved conditions within 3 days and not under normal or high glucose conditions9. However, oxygen consumption rate (OCR) data revealed that oxidative phosphorylation (OXPHOS) was more activated in normal glucose concentrations than in high glucose concentrations9. Considering this result, we investigated the effect of long-term CAP administration. We observed that the 7-day administration of CAP was more effective under normal glucose conditions than under high glucose conditions (Fig. 1a,b). Although cells died in normal glucose concentrations, this effect was decreased in high glucose concentrations, highlighting the importance of glucose control during treatment.
Effects of chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG) under different glucose conditions. (a) The effect of the 7-day administration of CAP in U87 (2 × 104 cells were seeded) under normal (1000 mg/L) and high (4500 mg/L) glucose conditions. CAP is effective under normal glucose conditions. (b) Cell number with CAP under glucose concentrations of 1000 and 4500 mg/L relative to the control shows that CAP is effective under normal glucose conditions (cells were seeded in a six-well plate and counted using a Coulter counter). (c) The effect of the 3-day administration of 2-DG in U87 under normal and high glucose conditions; 2-DG is effective at 1,000 mg/L glucose condition. (d) Cell number with 2-DG under normal and high glucose concentrations relative to the control shows that 2-DG is effective under normal glucose conditions (cells were seeded in a six-well plate and counted using trypan blue). Values are presented as mean ± standard deviation. Student’s t-test was performed under normal glucose vs. high glucose conditions. ***P < 0.001, ****P < 0.0001.
We subsequently used 2-DG because it inhibits glycolysis, mimicking glucose-starved conditions10. Moreover, 2-DG has been considered for treating cancers and solid tumors12,13. We expected 2-DG to inhibit cell growth alone or in combination with CAP. The 3-day administration of 2-DG decreased cell numbers under normal glucose concentrations (Fig. 1c,d). The effect of this drug was also decreased under high glucose conditions.
Combined treatment of 2-DG and CAP is effective
We hypothesized that the combined treatment of CAP and 2-DG is effective and, thus, assessed whether glioblastoma cells decreased after 3- and 5-day administration of these agents. Combined treatment was effective, and cell growth was inhibited in normal glucose conditions (Fig. 2a,b, Supplementary Fig. S1a–d). Moreover, cell count showed combination effects in CAP and 2-DG. However, under high glucose concentrations, the effectiveness of combined therapy decreased (Fig. 2c,d). This combined treatment is effective because 2-DG creates glucose-starved conditions.
Effects of combined treatment, including chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG), under different glucose conditions. (a) The effect of the 5-day administration of CAP and 2-DG in U87 under normal glucose conditions. The combined treatment was effective. (b) Cell number with each agent under a glucose concentration of 1000 mg/L (cells were seeded in a six-well plate and counted using a Coulter counter). (c) The effect of the 5-day administration of CAP and 2-DG in U87 under high glucose conditions. (d) Cell number with each agent under a glucose concentration of 4500 mg/L (cells were seeded in a six-well plate and counted using a Coulter counter). Values are presented as mean ± standard deviation. Statistical significance was assessed using the ordinary one-way analysis of variance test with Tukey’s multiple comparison test assessing Ct vs. CAP vs. 2-DG vs. CAP + 2-DG. **P < 0.01, ***P < 0.001, ****P < 0.0001.
2-DG increases COX1 expression and OXPHOS
COX1 expression increased after 2-DG administration (Fig. 3a,b). Therefore, we monitored the OCR to evaluate OXPHOS and found that the OCR was higher under 2-DG administration than the control and that the OCR decreased in the CAP group (Fig. 3c,d). Considering these results, 2-DG mimics glucose-starved conditions, increasing the effectiveness of CAP. Subsequently, we consider this nutrient-deprived condition leads to an increase in the activity of fatty acid oxidation to produce energy. To detect the OCR derived from fatty acid activation, etomoxir, a carnitine palmitoyltransferase 1A (CPT1A) inhibitor, was used18,19. OCR decreased when etomoxir was injected (Supplementary Fig. S2a,b).
2-Deoxy-d-glucose (2-DG) makes the cell’s mitochondria dominant and increases the oxygen consumption rate (OCR). (a) 2-DG increased the expression of COX1 in U87 after 3 days. (b) Quantification of COX1 expression (N = 3). Values are presented as mean ± standard deviation. Student’s t-test was performed on normal glucose vs. high glucose conditions. **P < 0.01. (c) Traces of OCR in each agent administered and (d) quantification of maximal respiration. Ordinary one-way analysis of variance with Tukey’s multiple comparison test was performed to assess Ct vs. CAP vs. 2-DG vs. CAP + 2-DG. *P < 0.05, **P < 0.01, ****P < 0.0001.
Combined treatment is effective even under hypoxic conditions
In the biological environment, cells exist in hypoxic and low-nutrient conditions14,15. We assessed whether the effectiveness of combined treatment under hypoxic conditions (O2 = 1% is sufficient for triggering a hypoxic response (Supplementary Fig. S3a,b) and found that cell growth was inhibited under normal glucose conditions (Fig. 4a,b). Moreover, the effects of CAP and 2-DG were positive effect; however, under high glucose concentration, their effectiveness was decreased (Fig. 4c,d).
Effects of combined treatment, including chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG), under different glucose conditions in hypoxia. (a) The effect of the 5-day administration of CAP and 2-DG in U87 under normal glucose conditions for in hypoxia (O2 = 1%). The combined treatment was effective. (b) Cell number with each agent under a glucose concentration of 1000 mg/L (cells were seeded in a six-well plate and counted using a Coulter counter). (c) The effect of the 5-day administration of CAP and 2-DG in U87 under high glucose conditions. (d) Cell number with each agent under a glucose concentration of 4500 mg/L (cells were seeded in a six-well plate and counted using a Coulter counter). Values are presented as mean ± standard deviation. Statistical significance was assessed using the ordinary one-way analysis of variance test with Tukey’s multiple comparison test assessing Ct vs. CAP vs. 2-DG vs. CAP + 2-DG. **P < 0.01, ***P < 0.001, ****P < 0.0001.
Combined treatment is effective when delivered within patient-derived neurosphere cells
Finally, we used patient-derived stem-like cells to validate the effects of treatment mimicking an in vivo condition. Although single administration of CAP or 2-DG was ineffective, combined treatment was effective in neurosphere cells (Fig. 5a,b). The neurospheres were smaller, and the number of spheres decreased (Supplementary Fig. S4a,b).
Effects of combined treatment in patient-derived stem-like cells. (a) The effect of the 7-day administration of chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG) in KNS1451 under normal glucose conditions. The combined treatment was effective. (b) Cell number with each agent under a glucose concentration of 1000 mg/L (cells were seeded in a six-well plate and counted using trypan blue). Values are presented as mean ± standard deviation. Statistical significance was assessed using the ordinary one-way analysis of variance test with Tukey’s multiple comparison test assessing Ct vs. CAP vs. 2-DG vs. CAP + 2-DG. **P < 0.01.
Combined treatment induces changes in iron dynamics
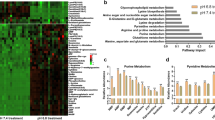
We investigated the mechanisms underlying the effectiveness of combined treatment. As previously reported, CAP injection results in cell death via ferroptosis under glucose-starved conditions9. Thus, we hypothesized that ferroptosis underlies the effectiveness of combined treatment. During CAP administration, the mRNA level of ferroptosis markers, including PTGS2 and CHAC1, increased (Fig. 6a,b, Supplementary Fig. S5a). Regarding the mRNA of HO-1 levels, one of the markers related to iron dynamics also increased in 2-DG administration (Fig. 6c). The mRNA levels of FTH1 increase upon treatment with either agent. However, the highest level of FTH1 mRNA was observed upon combined treatment, demonstrating the combination effect of these two agents (Fig. 6d, Supplementary Fig. S5b). In addition, the expression of ferroptosis-related proteins, including FTH1, GpX4, and KEAP1, demonstrated a combination effect (Fig. 6e–h). In particular, the expression of FTH1 and GpX4 increased using CAP and 2-DG. However, the expression of KEAP1 decreased using both CAP and 2-DG. These results imply that the two agents, CAP and 2-DG, cause drastic iron dynamic changes and that ferroptosis is one of the pathways that result in cell death. To exclude the possibility of other types of cell death, including apoptosis, we performed immunoblotting analysis to assess the expression of caspase-3, which implies apoptosis (Supplementary Fig. S6a,b). Furthermore, combined treatment with 2-DG and other ferroptosis inducers, namely sodium selenite, was also effective (Supplementary Fig. S7a,b)9,20.
Iron dynamics were changed drastically by both agents. A change in ferroptosis was observed after the 5-day administration of chloramphenicol (CAP) and 2-deoxy-d-glucose (2-DG) treatment in U87. (a,b) Quantification of PTGS2 and CHAC1 mRNA expression (N = 3). (c) Quantification of HO-1 mRNA expression (N = 3). (d) Quantification of FTH1 mRNA expression (N = 3). (e) Western blot reveals that FTH1 and GpX4 levels increased in both agents, whereas KEAP1 levels decreased in both agents (N = 3). (f–h) Quantification results. Values are presented as mean ± standard deviation. Student’s t-test (a–c,f) or ordinary one-way analysis of variance with Tukey’s multiple comparison test (d,g,h) was performed to assess Ct vs. CAP vs. 2-DG vs. CAP + 2-DG. *P < 0.05, **P < 0.01, ***P < 0.001.
Cell death is inhibited by deferoxamine
Considering the results of mRNA and protein expression, CAP and 2-DG may induce ferroptosis, emphasizing the effectiveness of combined treatment. To prove this, we used DFO, an iron chelator and inhibitor of ferroptosis. DFO inhibited the effects of CAP (Fig. 7a,b) and those of 2-DG and CAP with 2-DG (Fig. 7c–e). Considering these results, cell death caused by CAP and 2-DG was inhibited by DFO, and one of the mechanisms of these agents is ferroptosis.
Inhibition of ferroptosis by deferoxamine (DFO). (a,b) In U87, cell number assay reveals that chloramphenicol (CAP) and DFO (50 μM, added 72 h after CAP injection) reduced cell death after 5 days. Values are presented as mean ± standard deviation. (N = 3). (c–e) In U87, the cell number assay revealed that 2-deoxy-d-glucose (2-DG) or CAP + 2-DG and DFO reduced cell death after 3 days, wherein DFO (50 μM) was added initially. Values are presented as mean ± standard deviation. (N = 3). A Student’s t-test was performed. **P < 0.01, ***P < 0.001.
Discussion
Little is known about the relationship between glioblastoma and the mitochondria. We focused on the mitochondria because they are key organelle targets in treating glioblastoma21. In addition, hypoglycemic and hypoxic conditions exist in the tumor environment14,15. In our previous study, we focused on the development of glioblastoma treatment under hypoglycemic conditions9. The findings of this prior study were as follows: mitochondria were activated under glucose-starved conditions, and antimicrobial agents were effective under glucose-starved conditions. Because the tumor environment is hypoglycemic and deficient in various nutrients, previous findings represented promising treatments. However, under glucose-starved conditions, the glucose level (100 mg/L) was much lower than under normal glucose conditions (1000 mg/L)9. Considering tumor heterogeneity, some high-nutrient lesions may have higher glucose levels than low-nutrient lesions. It is important to develop an effective treatment with various oxygen concentrations in normal and low glucose conditions to overcome these malignant tumors. Therefore, in this study, we focused on normal glucose and hypoxic conditions and developed a combined treatment using CAP and 2-DG. This treatment inhibited cell growth drastically under normal glucose conditions in both normal and hypoxic conditions. Thus, combined treatment can be promising for glioblastoma patients.
According to our results, combined treatment was ineffective under high glucose (4500 mg/L) compared to normal glucose conditions, highlighting the importance of maintaining glucose levels within the normal range. According to a previous study, blood glucose levels are associated with prognosis in patients with glioblastoma, with higher glucose levels resulting in poorer prognoses22. In addition, the OCR was higher under normal glucose than under high glucose conditions, suggesting that mitochondria-targeted therapy is more effective under normal glucose conditions than under high glucose conditions9, with a glucose concentration of 1000 mg/L being considered normal in humans.
The blood–brain barrier (BBB) blocks some agents from invading the brain23. Thus, whether an agent can cross the BBB should be considered. Recently, nanotherapeutic techniques have been developed to overcome BBB to enhance glioblastoma treatment23. From various agents available, we selected two agents, CAP and 2-DG, that can easily cross the BBB and directly affect the mitochondria24,25.
CAP is an antimicrobial agent that induces mitochondrial dysfunction26. Moreover, this agent has been used to treat meningitis27. Dunkle et al.27 reported that the effective blood concentration for premature newborns with central nervous system infection was 46–154 μM. Furthermore, a 100 μM concentration is reasonable and less harmful. In addition, when using this agent, drug repositioning is expected. Metformin has recently been used to treat cancers, including glioblastomas28. Kim et al.28 reported that 2-DG combined with metformin inhibits glioblastoma cell proliferation. We investigated the effect of metformin under glucose-starved conditions, and our study reported that CAP was effective under glucose-starved conditions9, whereas metformin was not very effective (Supplementary Fig. S8). This difference is probably due to differences in the mechanisms of action of these two drugs. CAP inhibits mitochondrial ribosomes and causes mitochondrial dysfunction26. Metformin reportedly inhibits the mitochondrial complex 1; however, another theory has been proposed, and the exact mechanism has not been fully elucidated29. CAP is effective under both glucose-starved and normal conditions. Therefore, we believe that CAP is a key drug for treating glioblastoma.
2-DG is used to treat cancers targeting glycolysis12. Stein and Raez conducted a clinical trial to treat cancers or solid tumors using 2-DG and presented its safety at appropriate concentrations. Stein recommended a 45 mg/kg dose, whereas Raez recommended a safe dose of 63 mg/kg/day12,13. In addition, the Cmax of a 45 mg/kg dose is 73.7 μg/ml (449 μM), whereas that of a 63 mg/kg/day dosage is 116 μg/ml (almost 700 μM). However, Sasaki et al.30 reported that although 2-DG successfully treats cancer, effective doses induce serious adverse effects. They, therefore, recommended a new device that delivers 2-DG in poly-lactic-co-glycolic acid nanoparticles. In our study, the concentration of 2-DG was 300 μM, which is within the safe range.
2-DG inhibits glycolysis and provides conditions similar to glucose starvation. In glucose-starved conditions, as previously reported, mitochondria become dominant. 2-DG also increases the expression of COX1 and OXPHOS, implying mitochondrial dominance. Additionally, we consider that this nutrient deprivation leads to increased fatty acid oxidation activity and suppose the OXPHOS increase is due to fatty acid activations. Etomoxir is used to detect the activity of fatty acid oxidation, and it is reported that high dose etomoxir inhibits mitochondrial complex 131. OXPHOS decreased after the etomoxir injection, although the decrease was less than expected. Thus, there may be other pathways, including the amino acid pathway. Further investigation is needed to elucidate this.
Based on the study results, ferroptosis may have occurred. Several markers of ferroptosis, including KEAP1, NRF2, HO-1, GpX4, TFRC, FTH1, and xCT, have recently been reported17. A previous study has shown that CAP induces ferroptosis via the p-p62-KEAP1-NRF2-HO-1 pathway9. In this study, we used two different agents; it was, therefore, difficult to confirm the existence of a single ferroptosis stream. However, the two agents drastically affected iron dynamics and showed a combination effect on FTH1, GpX4, and KEAP1. Further, to confirm whether these agents induce ferroptosis, it is important to use an inhibitor of this pathway; we used DFO accordingly. DFO inhibited both agents, and we believe that the mechanism underlying cell death, in this case, is ferroptosis. In this study, we assessed other pathways of cell death, including apoptosis and necroptosis. The expression of caspase 3, which implies apoptosis, was not changed in each agent. Moreover, an increase in the level of RIP3K mRNA, which is related to necroptosis, was not detected. In addition, one reason 2-DG causes ferroptosis may be that glucose deprivation induces the blocking of the serine synthesis pathway, a pathway derived from glycolysis32. This leads to the depletion of glutathione, an activator of GpX4 protecting against ferroptosis33.
One limitation of this study is that data were collected in vitro rather than in vivo; thus, further in vivo studies on mice are required. However, the development of effective treatment is worth reporting. In conclusion, we developed an effective treatment combining CAP with 2-DG to treat glioblastoma cell lines under normal glucose conditions. Therefore, the results of our study seem beneficial for developing treatments for glioblastoma.
Methods
Cell culture
U87MG (U87) and U373 were obtained from the American Type Culture Collection (Manassas, VA, USA) (certified by BEX [Japan]). The use of cell lines for this study was approved by the Ethics Committee of the Graduate School of Medical Science, Kyushu University. Written consent was obtained from all patients.
The cells were cultured, as previously reported9. Similarly, an original patient-derived glioblastoma cell line, KNS1451, obtained from Kyushu University Brain Tumor Bank, was cultured as previously reported9. Genetic analysis revealed that KNS1451 harbored mutations, such as phosphatase and tensin homolog, tumor protein 53, neurofibromatosis 1, and telomerase reverse transcriptase promoter C250T. This cell line is classified as an aggressive mesenchymal type.
Quantitative real-time PCR
Real-time PCR was performed, as previously described9. Ribosomal 18S rRNA was evaluated as an internal control. Primer sequences are shown in Supplementary Table S1.
Immunoblotting analysis
Immunoblotting analysis was performed, as previously described9. The following antibodies were used in this study: mouse monoclonal anti-MTCO1 (#ab14705, Abcam, RRID:AB_2084810), mouse monoclonal anti-β-actin (#A5441, Sigma-Aldrich, RRID_2766243), rabbit polyclonal anti-GPX4 (#52455, CST, RRID:AB_2924984), rabbit monoclonal anti-KEAP1 (#8047, CST, RRID:AB_10860776), rabbit polyclonal anti-FTH1 (#3998, CST), rabbit polyclonal anti-Caspase-3 (#9662, CST), rabbit monoclonal anti-HIF-1α antibody (#14179, CST, RRID:AB_2622225), anti-rabbit IgG HRP-linked (#7074, CST), and anti-mouse IgG HRP-linked (#7076, CST). The membranes were cut prior to hybridization with antibodies.
Cell number counts
The cells were seeded (1 × 105 in a 6-well dish except for that in Fig. 1 in which 2 × 104 cells were seeded) in triplicate and cultured in DMEM (containing glucose, CAP (034-10572, Wako), 2-DG (D8375, Sigma-Aldrich), DFO (205-314-3, Sigma-Aldrich), SS (10102-18-8, WAKO), and metformin (1115-70-4, Tokyo Chemical Industry) at each concentration. This culture medium was replaced every 3 days; the cells were trypsinized and counted using a Coulter counter (Beckman Coulter, USA) or TC 20 automated cell counter (BIO-RAD, #1450101J1, USA) with trypan blue. To assess the proliferation of stem-like cells, the cells (5 × 104 in a 6-well dish) were seeded in triplicate or more and cultured in DMEM/Ham’s F12 (containing CAP and 2-DG at each concentration) for 7 days. On the 7th day, the sphere number and size were evaluated. After centrifugation at 3000×g for 2 min, the cell pellets were trypsinized, added to the medium, and counted using the TC 20-cell automated cell counter with trypan blue.
Hypoxic experiments
Hypoxic conditions were achieved using a personal CO2 multi-gas incubator (ASTEC) with 1% O2 and 5% CO2. The O2 concentration was automatically checked by a Gas Cylinder Auto Changer (model 8420, Waken).
Seahorse XF24 flux analyzer
Mitochondrial OXPHOS and glycolytic activity can be measured using the OCR methods with an XFe24 analyzer (Seahorse Biosciences, USA). Basal OCR was measured using the Seahorse XF24 Flux analyzer, as previously described9. In addition to this, fatty acid activity can be measured using 40 μM etomoxir (#1905, sigma-aldrich) and 0.5 mM l-carnitine (#541-15-1, TCI)18,19. Seahorse XF24 microplates were seeded with 1 × 105 cells/well (before seeding, cells were incubated for 2 days under each condition) and incubated at 37 °C for approximately 16 h. After analysis, the cells were seeded with a similar number of cells in another 96-well plate, trypsinized, and counted at the start of the analysis. The results were normalized to the number of cells.
Statistical analyses
Statistical analyses are described in the figure legends. Data are presented as mean ± standard deviation. Significant differences between groups were examined using a one-way analysis of variance or Student’s t-test with GraphPad Prism version 9 (GraphPad Prism Software Inc.). All experiments were repeated at least thrice.
Data availability
All data generated or analyzed during this study are included.
References
Bao, Z. et al. High glucose promotes human glioblastoma cell growth by increasing the expression and function of chemoattractant and growth factor receptors. Transl. Oncol. 12, 1155–1163 (2019).
Ostrom, Q. T. et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 17, iv1–iv62 (2015).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Chen, W. et al. Optimal therapies for recurrent glioblastoma: A Bayesian network meta-analysis. Front. Oncol. 11, 641878 (2021).
Jiapaer, S., Furuta, T., Tanaka, S., Kitabayashi, T. & Nakada, M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol. Med. Chir. (Tokyo) 58, 405–421 (2018).
Wallace, D. C. Mitochondria and cancer: Warburg addressed. Cold Spring Harb. Symp. Quant. Biol. 70, 363–374 (2005).
Vyas, S., Zaganjor, E. & Haigis, M. C. Mitochondria and cancer. Cell 166, 555–566 (2016).
Ashton, T. M., McKenna, W. G., Kunz-Schughart, L. A. & Higgins, G. S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 24, 2482–2490 (2018).
Miki, K., Yagi, M., Yoshimoto, K., Kang, D. & Uchiumi, T. Mitochondrial dysfunction and impaired growth of glioblastoma cell lines caused by antimicrobial agents inducing ferroptosis under glucose starvation. Oncogenesis 11, 59 (2022).
Zhang, D. et al. 2-deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 355, 176–183 (2014).
Singh, D. et al. Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose escalation studies in patients with glioblastoma multiforme. Strahlenther. Onkol. 181, 507–514 (2005).
Stein, M. et al. Targeting tumor metabolism with 2-deoxyglucose in patients with castrate-resistant prostate cancer and advanced malignancies. Prostate 70, 1388–1394 (2010).
Raez, L. E. et al. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 71, 523–530 (2013).
Tanaka, K. et al. Glioma cells require one-carbon metabolism to survive glutamine starvation. Acta Neuropathol. Commun. 9, 16 (2021).
Junttila, M. R. & de Sauvage, F. J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501, 346–354 (2013).
Matsumoto, T. et al. Doxycycline induces apoptosis via ER stress selectively to cells with a cancer stem cell-like properties: Importance of stem cell plasticity. Oncogenesis 6, 397 (2017).
Li, J. et al. Ferroptosis: Past, present and future. Cell Death Dis. 11, 88 (2020).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Kim, S. J. et al. Dual inhibition of CPT1A and G6PD suppresses glioblastoma tumorspheres. J. Neurooncol. 160, 677–689 (2022).
Subburayan, K., Thayyullathil, F., Pallichankandy, S., Cheratta, A. R. & Galadari, S. Superoxide-mediated ferroptosis in human cancer cells induced by sodium selenite. Transl. Oncol. 13, 100843 (2020).
Iranmanesh, Y. et al. Mitochondria’s role in the maintenance of cancer stem cells in glioblastoma. Front. Oncol. 11, 582694 (2021).
Tieu, M. T. et al. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J. Neurooncol. 124, 119–126 (2015).
Tang, L., Feng, Y., Gao, S., Mu, Q. & Liu, C. Nanotherapeutics overcoming the blood-brain barrier for glioblastoma treatment. Front. Pharmacol. 12, 786700 (2021).
van de Beek, D., Brouwer, M. C., Thwaites, G. E. & Tunkel, A. R. Advances in treatment of bacterial meningitis. Lancet 380, 1693–1702 (2012).
Shah, S. S. et al. Targeting glioblastoma stem cells with 2-deoxy-D-glucose (2-DG) potentiates radiation-induced unfolded protein response (UPR). Cancers 11, 159 (2019).
Li, C. H., Cheng, Y. W., Liao, P. L., Yang, Y. T. & Kang, J. J. Chloramphenicol causes mitochondrial stress, decreases ATP biosynthesis, induces matrix metalloproteinase-13 expression, and solid-tumor cell invasion. Toxicol. Sci. 116, 140–150 (2010).
Dunkle, L. M. Central nervous system chloramphenicol concentration in premature infants. Antimicrob. Agents Chemother. 13, 427–429 (1978).
Kim, E. H. et al. Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro. Oncol 19, 197–207 (2017).
Feng, J. et al. Mitochondria as an important target of metformin: The mechanism of action, toxic and side effects, and new therapeutic applications. Pharmacol. Res. 177, 106114 (2022).
Sasaki, K. et al. Nanoparticle-mediated delivery of 2-deoxy-D-glucose induces antitumor immunity and cytotoxicity in liver tumors in mice. Cell. Mol. Gastroenterol. Hepatol. 11, 739–762 (2021).
Yao, C. H. et al. Identifying off-target effects of etomoxir reveals that carnitine palmitoyltransferase I is essential for cancer cell proliferation independent of beta-oxidation. PLoS Biol. 16, e2003782 (2018).
Amelio, I., Cutruzzola, F., Antonov, A., Agostini, M. & Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 39, 191–198 (2014).
Ursini, F. & Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 152, 175–185 (2020).
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers (JP20H00530, JP21K11678, JP22H03537).
Author information
Authors and Affiliations
Contributions
K.M. experiment, data curation, data analysis, methodology, and manuscript writing revision; M.Y. methodology, supervision, resources, and funding; N.N. and R.O. provision of clinical specimen; Y.D. supervision; D.K. supervision and provision of clinical specimen; D.K. methodology, supervision, resources, and funding; K.Y. conceptualization, methodology, supervision, and revision; T.U. conceptualization, supervision, resources, funding, and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miki, K., Yagi, M., Noguchi, N. et al. Induction of glioblastoma cell ferroptosis using combined treatment with chloramphenicol and 2-deoxy-d-glucose. Sci Rep 13, 10497 (2023). https://doi.org/10.1038/s41598-023-37483-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-37483-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.