Abstract
Surgical data quantification and comprehension expose subtle patterns in tasks and performance. Enabling surgical devices with artificial intelligence provides surgeons with personalized and objective performance evaluation: a virtual surgical assist. Here we present machine learning models developed for analyzing surgical finesse using tool-tissue interaction force data in surgical dissection obtained from a sensorized bipolar forceps. Data modeling was performed using 50 neurosurgery procedures that involved elective surgical treatment for various intracranial pathologies. The data collection was conducted by 13 surgeons of varying experience levels using sensorized bipolar forceps, SmartForceps System. The machine learning algorithm constituted design and implementation for three primary purposes, i.e., force profile segmentation for obtaining active periods of tool utilization using T-U-Net, surgical skill classification into Expert and Novice, and surgical task recognition into two primary categories of Coagulation versus non-Coagulation using FTFIT deep learning architectures. The final report to surgeon was a dashboard containing recognized segments of force application categorized into skill and task classes along with performance metrics charts compared to expert level surgeons. Operating room data recording of > 161 h containing approximately 3.6 K periods of tool operation was utilized. The modeling resulted in Weighted F1-score = 0.95 and AUC = 0.99 for force profile segmentation using T-U-Net, Weighted F1-score = 0.71 and AUC = 0.81 for surgical skill classification, and Weighted F1-score = 0.82 and AUC = 0.89 for surgical task recognition using a subset of hand-crafted features augmented to FTFIT neural network. This study delivers a novel machine learning module in a cloud, enabling an end-to-end platform for intraoperative surgical performance monitoring and evaluation. Accessed through a secure application for professional connectivity, a paradigm for data-driven learning is established.
Similar content being viewed by others
Introduction
Incorporating artificial intelligence (AI) powered by cloud connectivity to aggregate data in and across operating rooms (OR) offers an objective tool for systematic feedback on the optimal use of medical devices and systems. This is important for improving the safety of surgery and utilizing digital innovation towards standardization of patient care. Implementing AI through sensor-enabled and data-driven surgical devices can transform traditional and subjective training based on apprenticeship into an objective and non-intimidating paradigm1. Context-aware assistance by surgical phase recognition can further facilitate and improve the training process through particularized analytic feedback on the performance of surgery2. As a new frontier in surgical coaching, surgical data science can be defined through novel frameworks involving collection, structuring, analysis, and modeling of such data3,4.
Machine learning algorithms in surgery, while early, may enhance care in various pathologies, including epilepsy, brain tumors, spine lesions, and cerebrovascular disorders5. Sensor-driven data can be used to accurately capture surgeon dexterity and technical skill, using meaningful features extracted from surgical maneuvers and workflow. This, in turn, would help provide a quantitative feedback metric during a graduated surgical training period. The movement of instruments has been used in the past as a kinematic measure of performance and skill discrimination in a laboratory environment6,7,8. For skill evaluation, a deep learning-based instrument tracking system based on surgical videos has been implemented, which is compliant with Objective Structured Assessment of Technical Skill (OSATS) and Global Evaluative Assessment of Robotic Skill (GEARS) manual metrics9. Surgical skill assessment and navigation in colorectal surgery can be facilitated through forceps type and object recognition on video data10. Additionally, the use of motion features extracted from video temporal pattern analysis led to the categorization and analysis of surgical actions11,12. A comprehensive review of surgical skill analysis literature has also been published13. The manuscripts included in this review used kinematic (61%) and video (29%) data, with limited attention to tool-tissue forces14,15. The ML models used herein were Artificial Neural Networks (ANN), Hidden Markov Models (HMM), and Support Vector Machines (SVM), all with higher accuracies than 80%. Their findings, however, were limited in data from real-life surgery (12%), as well as the lack of a framework application for providing surgeons with interpretable and clinically relevant feedback.
Among the sensory data, kinesthetic force feedback, i.e., concerning the reconstruction of the human sense of touch by activating muscular mechanoreceptors, is eminent. This type of force can have implications for surgical outcomes, e.g., non-optimal force application leading to tissue damage or prolonged surgical times1,16,17. In various studies, grip force was used as a metric for assessing surgical skill6,18. Instrument force analysis showed a lower force level in experienced surgeons than novices when performing dry laboratory exercise6. Furthermore, a regression analysis for automated skill evaluation based on contact force with task materials, robotic instrument accelerations, and task completion time was also performed18. The findings were in agreement with the manual GEARS metric. In addition, the combination of visual signals with force feedback has been shown to enhance tissue characterization19 with lower force peak magnitudes leading to significantly lower tissue trauma and surgical error rates20. Previous studies while successful in their respective goals, never focused on performance evaluation based on surgical tasks, e.g., coagulation as a paramount aspect of vascular surgery, using a single modality data from tool-tissue interaction, i.e., forces16.
Here we present an original machine learning framework, i.e., from data ingestion, analytics, and machine learning, to insights, for information extraction using a data-rich environment enabled by a sensorized bipolar forceps coupled to an intelligent software platform, the SmartForceps System1,16,21,22,23,24. The medical grade SmartForceps are sterilized between each procedure following the standards approved by regulatory bodies and the Central Sterilization and Reprocessing Department. For regulatory approval, we have demonstrated that each SmartForceps withstands multiple cycles of sterilization without impacting the instrument’s sensors, i.e., altering the calibration1,25. This novel framework builds upon our recent work on a data-enabled surgical performance dashboard, now creating an automated analytical platform. The work leverages sensor-based technology whereby evolving AI systems complement the way surgery is performed and taught. The modeling efforts encompass deep learning architectures and data analytics for surgical skill classification between Expert and Novice, and the recognition of a critical neurosurgical task, i.e., Coagulation, to improve granularity in performance feedback. Such analytics on surgical performance and comparison to the gold standard can be reviewed in an interactive environment, i.e., the Expert Room. This study offers new opportunities within an objective and sensor-driven surgical performance tracking and analytics model, towards improved learning and safety of surgery.
Materials and methods
Data recording
The SmartForceps System (developed at Project neuroArm, University of Calgary, Calgary AB, Canada) allows real-time display and recording of tool-tissue force data during surgery. Surgical tasks were categorized into: (1) Coagulation (cessation of blood loss from a damaged vessel), (2) non-Coagulation with sub-categories of (a) Dissection (cutting or separation of tissues), (b) Pulling (moving and retaining tissues in one direction), (c) Retracting (grasping and retaining tissue for surgical exposure), and (d) Manipulating (moving cotton or other non-tissue objects), which were identified following expert approval of cumulative data reviews. The audiotaped voice of each surgeon accompanied force recordings, which indicated the periods of force application and specific task names. This information facilitated the labeling process for each force segment, creating a supervised dataset for the machine learning models. The study was approved by the Conjoint Health Research and Ethics Board of the University of Calgary, Calgary, AB, Canada (REB19-0114), with the technology approved by Health Canada (ITA 329,641 Class II, 2021). Details on technology development, pre-clinical and clinical use have been previously published16,21,22,23,26,27. Informed electronic and verbal consent was obtained from participating surgeons per the REB, which included a waiver of informed patient consent by the Institutional Review Board at the University of Calgary. The surgical team adopted the SmartForceps system in place of the conventional bipolar forceps with the added advantage of real-time tool-tissue force measurement, display, and recording. Adult patients undergoing elective surgical treatment for various intracranial pathology were included in this prospective study (under the supervision of the senior author as the staff surgeon). Emergency neurosurgical procedures and the pediatric population were excluded. No identifiable patient information is included in the manuscript and methods were performed in accordance with the relevant guidelines and regulations for human experimental studies and Declaration of Helsinki.
The data framework included a HIPAA and PIPEDA Compliant Cloud architecture for retaining and processing the intraoperative de-identified data through a Cloud platform (Microsoft Azure, Microsoft USA) with secure authentication through organizational credentials. In addition, an installable web/mobile application was developed to monitor the force-related data/features, which is available at smartforceps-app.azurewebsites.net.
Workflow architecture
To quantify the behavior of force profiles for pattern recognition and performance analysis, we developed machine learning models for segmenting and recognizing the patterns of intra-operative force profiles. The models make no assumption about the underlying pattern in force data and hence are robust to noise. The framework enables modeling a complex structure in our non-stationary time-series data, where data characteristics including mean, variance, and frequency change over time. Figure 1 shows the workflow architecture from data recording to modeling and visualization.
Workflow architecture of SmartForceps platform from data recording to modeling and visualization. Forces of tool-tissue interaction along with de-identified case information were uploaded to a HIPAA-compliant data storage and analytics platform. Force data were manually segmented and labeled by listening to the surgeon’s voice recordings, where surgeon names, surgical tasks, and important incidents were narrated. The AI modeling architecture included Auto Data Preprocessing (e.g., Data Balancing, Outlier Removal, Data Transformation, etc.), Feature Engineering, Data Modeling (T-U-Net for force profile segmentation (T-U-Net: Time-series-U-Net); XGBoost, LSTM and FTFIT (Force Time-series Feature-based InceptionTime) for pattern recognition), and Modeling Optimization and Performance Evaluation, which were integrated into the cloud platform to generate performance evaluation reports to the surgical team. A detailed description of selected processes in the figure has been described in the Supplementary Materials. Visualization was created in Microsoft PowerPoint version 16.49 with the icons obtained from a Google search: e.g., https://www.iconfinder.com.
Force profile segmentation
The force data points labeled as ON or OFF were included for analysis after applying rule-based data balancing (Figure S1, detailed in Supplementary Materials). To transform data to a normal distribution, i.e., Gaussian with zero mean and unit variance, and to eliminate the dominant effect of larger variance in a specific signal, feature normalization of the left and right prong force was performed by removing the mean and scaling to unit variance. This allowed standardization of corresponding values. Data preparation comprised of normalization and reshaping into windows of 224 data points which ended up with approximately 5.9 K resampled force windows. Following this, the segment labels were encoded, and a one-hot encoding schema was implemented. Finally, a 80% (20% validation)—20% split with a random seed was performed to split the data into training-validation and testing samples. A custom-designed U-Net (T-U-Net: Time-series-U-Net; U-Net is a dominant model for image segmentation28) model was trained and implemented that consisted of a convolutional encoder and decoder structure to capture the properties and reconstruct the force profile. Grid search was performed for hyperparameter tuning (Figures S2, S3, detailed in Supplementary Materials).
Surgical force pattern recognition
Data pre-processing for surgeon skill classification
Segmented training data with binary Expert and Novice labels were included in this phase. To fortify prediction power in force data, a total of 29 hand-crafted features (Table S1) that can capture the behavior of surgical force time-series data and were analyzed in our previous study1 were calculated for each window of 200 data points and were added as the third signal to a deep learning model after a feature selection process (Supplementary Materials Tables S2, S3). Our data curation pipeline performed time-series based feature extraction on the segmented data after noise reduction1. The normalization process transformed the feature data into a Gaussian distribution with zero mean and unit variance and resampling to match the force data window size of 200 points with ratio to maximum as the order of spline interpolation and edge mode for the boundary data imputation. The normalized and reshaped data created 3.6 K resampled force segment windows (1766 Novice and 1859 Expert segments), which were encoded using a one-hot vector and a random split into training-validation, i.e., 80% (20% validation), and testing, i.e., 20%, samples.
Data pre-processing for surgical task recognition
In this phase, the main surgical task of Coagulation was considered as a data label to be distinguished from other tasks. Similar to the skill classification model, the 29 hand-crafted features (Table S1) were fed into the neural network after being calculated over 200 data point window, proper noise reduction, outlier removal, normalization, and resampling, and feature selection (Supplementary Materials Tables S4, S5). The processed force segments comprised of 2 K samples (1170 force segments of Coagulation and 915 segments of non-Coagulation (Manipulation = 323, Pulling = 316, Retracting = 149, and Dissecting = 127)) with one-hot encoding format having 64% training, 16% validation, and 20% testing samples.
Model implementations
Two deep learning and a baseline model were created to classify surgeon experience levels (i.e., Novice and Expert) and activity recognition while performing a specific task (i.e., Coagulation and non-Coagulation (e.g., Pulling, Manipulation, Dissecting, and Retracting)). A deep neural network model for time series classification based on InceptionTime29, i.e., FTFIT (Force Time-series Feature-based InceptionTime), was developed to obtain the learned features. This, together with engineered features described above, was used in a logistic regression-based surgeon experience classification. A second deep learning model based on an LSTM neural network for time-series-based surgeon activity and experience recognition was used. These models followed a baseline XGBoost classifier that used the hand-crafted features (details of the modeling and results are in the Supplementary Materials Figures S11, S12 and S18, S19). Further details on model characteristics and hyperparameter tuning are available in Supplementary Materials (Figures S4, S5, S6).
Modeling evaluations
For all models, summary including the type, shape, and parameter counts for each layer; loss and accuracy values for both training and validation data in each epoch; classification report including fivefold cross-validation accuracy, selected model (through grid search on validation loss) testing accuracy (sensitivity and specificity), average precision, recall, weighted F1-score, and area under the curve (AUC) for receiver operating characteristic (ROC), and precision-recall curves during validation and testing with the corresponding charts and graphs were generated. Model training was performed using a workstation with Intel Core i9-9820X (10 cores, 4.20 GHz turbo) CPU, 2 × Titan RTX with NVLink GPU, and 64 GB memory taking approximately 0.7 h for the training and validation of data segmentation and 0.4 h for skill classification and task recognition models.
Results
Tool-tissue interaction force data from 50 neurosurgery procedures of adult tumor resection (30 males/20 females, mean (SD) age: 54.7 (14.1)) between November 2019 and October 2020, including meningioma (n = 10), glioma (n = 10), schwannoma (n = 15), and hemangioblastoma (n = 3) (+ 12 other cases, e.g., trigeminal neuralgia/hemifacial spasm, cavernous angioma, etc.) was employed. The cases were performed by 13 surgeons, i.e., one Expert with 30 + years of experience and twelve Novice surgeons, including residents with post-graduate years (PGY) ranging across three levels of 1–2 (n = 4), 3–4 (n = 3), and > 4 years (n = 4), and one fellow.
Force profile segmentation
Point-wise data classification as ON and OFF regarded as segments of force data through T-U-Net model showed the best results for 0.001 learning rate, 16 as filter size, moving window size of 224, and batch size of 128. The mean inference time was 0.24 s, and the minimum validation loss value occurred at epoch 27 (Figure S7a) was 0.1046 (training loss = 0.0853). fivefold cross-validation results showed a mean (SD) accuracy of 0.95 (0.01). Macro-AUC of ROC was 0.99 and when testing the model, accuracy was 0.95 (F1-score: 0.96 for class ON, and 0.95 for class OFF, weighted value = 0.95) (Table 1). Detailed results are illustrated in Figure S7, S8, S9, S10 (Supplementary Materials).
Surgical skill classification
The overlapping distribution of features in Expert and Novice classes is an early sign for a sub-optimal performance of feature augmentation to the network (Fig. 2a). Time-series classification performed best in FTFIT with no hand-crafted features added to the network (AUC = 0.81; p value < 0.001) (Fig. 3a). The model was characterized by a learning rate of 0.001 and a network depth size of 6, moving window size of 200, and batch size of 128. Testing time for each sample occurred in an average of 0.24 s, and the model reached minimum validation loss at epoch 66 (out of 100 epochs) (validation loss = 0.5285 and training loss = 0.4841 (Figure S14a)). Macro-AUC of ROC was 0.81 and while testing the model for unseen instances of force data, the accuracy was 0.71 (mean (SD) value of fivefold cross-validated accuracy was 0.73 (0.03)) with F1-score of 0.71 in both Expert, and Novice classes (weighted value = 0.71) (Table 1). Detailed results are available in Figures S14, S15, S16, S17 (Supplementary Materials).
-
(a)
Skill classification model The figure shows the shape of standardized (Gaussian with zero mean and unit variance) data distribution for each skill, i.e., normal with a low tendency of negative skewness in Entropy, normal with a low tendency of positive skewness in Range Force, negative skewed in Heterogeneity, and positively skewed in Duration Force. In addition, a positive correlation in Stability vs. Range Force and a negative correlation in Entropy vs. Range Force and Stability vs. Entropy was observed. Note: data visualization was created after outlier removals of Z-score < 3 across the samples.
-
(b)
Task recognition model The shape of standardized data distribution for coagulation vs. other tasks, i.e., normal in Entropy, negatively skewed in Heterogeneity, and positively skewed in Duration Force and Range Force has been shown. In addition, a positive correlation was noted in Heterogeneity versus Range Force and a negative correlation in Entropy versus Range Force and Heterogeneity versus Entropy. Note: data visualization was created after outlier removals of Z-score < 3 across the samples.
Performance comparison between LSTM and FTFIT models with hand-crafted feature combinations in surgical skill prediction and task recognition. Different combinations of hand-crafted features, i.e., no feature, selected set of features identified through KNN and XGBoost feature importance ranking, and a subset of features (Duration Force, Range Force, Entropy, and Heterogeneity; which were consistent with the features presented in SmartForceps performance dashboard1) have been compared.
(a) Surgical skill prediction The best performing model was FTFIT with no hand-crafted features added to the network showing an AUC = 0.81 (p value < 0.001).
(b) Surgical task recognition FTFIT with subset 1 of the hand-crafted features (n = 4) added to the network was among the best performing models with an AUC = 0.89 (p value < 0.001).
Surgical task recognition
Performance of task recognition for Coagulation and non-Coagulation (after a random selection of 0.5 Coagulation segments for data balancing) using FTFIT with subset 1 of the hand-crafted features (n = 4) added (Fig. 2b) to the network was among the best results (AUC = 0.89; p value < 0.001) (Fig. 3-b). This model had a learning rate of 0.01, a network depth size of 12, a moving window size of 200, batch size of 128, and concluded with a mean inference time of 0.20 s. This model's minimum validation loss value occurred at epoch 46 (out of 150 epochs) with validation loss of 0.4002 and training loss of 0.3025 (Figure S21a). Macro-AUC of ROC was 0.89 and testing results showed 0.82 in accuracy with a mean (SD) fivefold cross-validated accuracy of 0.79 (0.07). (F1-score of the Coagulation class was 0.85 and for non-Coagulation it was 0.77; weighted average = 0.82) (Table 1). Detailed results are available in Figure S21, S22, S23, S24 (Supplementary Materials).
End-to-end pipeline implementation
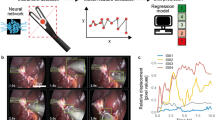
Machine learning models were translated to resources and pipelines embedded in the cloud platform for on-the-fly analytics and feedback to surgeons. Final output for segmentation and skill/task recognition was visualized through comparative distribution plots and individual force profile segments as previously described1. Figure 4 shows the force profiles and performance report of a surgeon across 3 cases of brain tumor resection.
Overview of the processed data for three surgical procedures using SmartForceps machine learning modeling and recognition. This figure is a snapshot of the Surgical Force Data tab in the SmartForceps performance monitoring dashboard showing the final output of the data and analytics pipeline. The pipeline started from operating room data collection using dedicated software, continued with Microsoft Data Factory running analytics engine for preprocessing data and pattern recognition, and ended with the mobile/desktop performance monitoring dashboard.
(a) Surgical force data In this representation, aggregative data distribution of both Expert-level (green violin plots) and Novice-level (purple violin plots) performance of the surgeon across the surgical tasks based on force Uncertainty Index (i.e., Entropy as a feature) selected from the dropdown menu (left column chart) is reported. The right column chart shows actual force profiles for the left (red time-series plot) and right (blue time-series plot) prong of SmartForceps.
(b) Performance comparison This figure shows performance comparisons (purple bar) of the surgeon compared to the Expert level surgeons (mean and standard deviation indicated as red mark and green area, respectively) after surgical-force-related feature extraction on segmented force profiles by T-U-Net. The gauge charts show the respective values for Average Force Duration, Range of Forces, Force Variability, and Force Uncertainty Indexes across 3 surgical procedures performed by the surgeon. In this graph, the representative surgeon gauge starts from zero as the baseline with the goal of reaching to the expert level values denoted by a red bar and green area. This surgeon had a higher average force duration (2.19 s more), lower range of forces (0.2 N less), lower force variability (46 points less), and higher force uncertainty (0.12 points more) compared to the average of expert data.
Discussion
This work presents an original algorithm running in perpetuity behind-the-scenes in the OR as a data-enabled virtual surgical assistant in real-world settings30,31. Built upon the time-series data obtained using SmartForceps, a step-by-step process was developed to establish unique machine-learning models custom-tailored for real-time credible performance feedback and interconnectivity in the OR. Indeed, such automated analytics based on tool-tissue interaction force provided a holistic view of combinatorial elements shaping surgical skill, e.g., tool-tissue forces, force profile, motion, hand–eye coordination, etc., all of which may contribute to surgical finesse1,32.
In training a machine learning framework, any data imbalance can pose a challenge in the predictive models through bias toward the majority class. This problem was mitigated through data elimination, i.e., force block removal during device-idle periods in force profile segmentation, and random 50% sampling of force segments in the high frequent task of Coagulation. Since the time-series segmentation model provides a point-wise classification of ON/OFF instances, post-processing analytics are necessary for the production phase, including extracting continuous force-ON blocks meeting data length requirements and reconciling the noise-driven discontinuity in the identified segments. Production pipeline incorporated data factories, functions, and REST APIs that, following the upload of OR data into the cloud, served as personalized performance monitoring dashboard application1,27.
While annexing hand-crafted features to neural networks for skill recognition and task classification models was preferred, the output can imply dual inference. Following extensive trials, it was evident that a selected feature-set incorporation would reduce performance in skill classification (AUC reduces from 0.81 to 0.76 in FTFIT when using the window size of 200). On the other hand, the combination of replicated performance features in the SmartForceps monitoring dashboard1, e.g., Duration Force, Range Force, Entropy, and Heterogeneity, were among the best performing combinations in deep learning models (performance range from AUC = 0.85 to 0.89 in task recognition models using LSTM or FTFIT). This showed the importance of application-specific optimal modelling and its validation for usage in the real world. In machine learning models with limited data, overfitting occurs frequently, and the baseline models indicate such a phenomenon as shown in the Supplementary Materials with the differences seen between training and testing accuracies. To mitigate overfitting, grid search for model fine tuning and early stopping based on validation loss were implemented. A fivefold cross-validation was performed to assess the final performance of the model based on the best hyperparameters. The results showed good matching between testing and mean (SD) cross-validated accuracies for segmentation (0.95 vs. 0.95 (0.01)) and skill classification (0.71 vs. 0.73 (0.03)), but higher variabilities for task recognition (0.82 vs. 0.79 (0.07)). However, an accuracy of 0.7 is the minimum value that is covered across all the cross-validated models.
Although included feature sets went through a normalization process before circulating in the deep neural networks, some of these features inherent variability and noise-prone characteristics (e.g., Spikiness and Coefficient of Variance with very low and high variabilities, respectively) would negatively affect a time-series profile descriptor. The distribution of feature values in Fig. 2a, despite Fig. 2b for task categories, showed high similarity across skill levels. This is, to some extent, reflected in performance comparison between the two pattern recognition efforts, i.e., task recognition has a better performance than skill classification. This suboptimal performance of surgical skill classification can be explained through a statistical analysis of the factors underlying this model. Our analysis showed that the mean (SD: Standard Deviation) for Force Duration in Coagulation was 12.1 (7.2) seconds (i.e., around 58% higher than the average of completion time in other tasks: two-way ANOVA test p value < 0.001), however, this measure comparison across Expert and Novice groups was 12.2 (7.2) versus 12.1 (7.3) only 0.8% difference. Similar behavior was observed for Minimum Force (Task Classes p value = 0.1; Skill Classes p value < 0.001), Force Distribution Skewness (Task Classes p value = 0.8; Skill Classes p value < 0.001), and Force Profile First Autocorrelation Zero (Task Classes p value = 0.9; Skill Classes p value < 0.001)13. A lower performance for skill classification has also been reported in previous studies where investigators showed a mean precision of 91% in detecting surgical actions, however, 77% when predicting surgical skills using deep learning on surgical videos 6. This may in part relate to real-world scenario whereby trainee surgeons perform only those tasks delegated by the attending based on their level of competency and comfort. In addition, this similarity of pattern can perhaps be attributed to the trainees following the mentor’s lead in our single institutional data. Including multi-institutional data with more distinctive patterns across mentor-trainee populations and procedures would help equip and enrich the machine learning framework with more granularity and diversity of incoming data, i.e., rating of skill proficiency, into the skill level classification model.
Of interest, the input time-series window size had an impact on modeling performances, i.e., AUC = 0.78 to 0.81 for the skill classification model and AUC = 0.87 to 0.89 for the task recognition model using the FTFIT network. This primarily related to the average duration time of a force segment, which was close to 10 s (200 data points considering the sampling rate of 20 Hz). Internalization of the FTFIT network for SmartForceps data modeling showed significant improvement compared to a widely used deep learning model, i.e., LSTM, for both skill recognition (AUC improvements from 0.60 to 0.81) and task recognition (AUC improvements from 0.69 to 0.89). This comparison with the baseline XGBoost model also showed an improvement in testing accuracy, i.e., from 0.65 to 0.71 in skill classification and from 0.81 to 0.82 in task recognition. Convolutional operations in FTFIT further allowed the local structure of force profile, e.g., line and curves, to be captured in bottom-layer neurons of the network, while various shapes, e.g., valleys and hills, in the top layers. Additionally, the speed performance, and scalability establish FTFIT as a suitable candidate for widespread use of the SmartForceps machine learning platforms29.
Efforts in utilizing AI for surgical monitoring and performance assessment have been initiated, mainly in surgical robotics, linking haptic feedback, robot kinematics, and clinical information such as operating time, blood loss, etc. to predict surgical outcomes as a measure of performance33,34. Similarly, surgical video-based localizing of surgical instrument’s trajectory and motion characteristics, using the Fast R-CNN model have been employed for estimation of performance monitoring35. For surgical task recognition, investigators have studied continuous kinematic data represented as strings for discriminative gesture discovery via relative occurrence frequency measured by comparative numerical statistics36. Low-level spatiotemporal features from video data, combined with a high-level segmental classifier based on a convolutional neural network integrating visual objects with temporal components have also been used37. Data-driven approaches have been developed for clinical decisions support. Multi-source preoperative and intraoperative data from a large number of surgical cases were used to predict postoperative complications38. Additionally, deep learning was used to forecast surgical duration in real-time for informed preoperative decisions39. Although one-to-one comparison of performance between studies was deemed unsuitable due to differing goals, a single modality, force data, has never been used to evaluate performance based on surgical tasks, such as coagulation as a vital component of vascular surgery.
Limitations
This study was limited by the inclusion of only one Expert surgeon, which may affect the surgical skill classification model. As the technology currently spreads to other centers, it allows a diversified data collection from a variety of surgical teams. Clinical trials and retraining of models with larger numbers of surgical teams will be included in future studies.
Conclusions
Here, using the SmartForceps technology, we have developed a unique end-to-end data-enabled pipeline that consolidates the concepts of immortalizing surgical skills. Perhaps this could be considered as a virtual assist hosted in a cloud platform enabling access beyond geographical or generational limits. Facilitating contemporary transition to competency-based surgical education and practices, sensor-driven technology, which allows a digital, quantifiable output, is deemed timely and necessary.
Additional information
Supplementary figures and table are available for this paper through this link: https://github.com/smartforceps/ai_models/tree/main/supplementary-files.
Data and code availability
Sample de-identified data and modeling codes are available at a GitHub repository: https://github.com/smartforceps/ai_models.
References
Baghdadi, A. et al. A data-driven performance dashboard for surgical dissection. Sci. Rep. 11(1), 1–13 (2021).
Padoy, N. et al. Statistical modeling and recognition of surgical workflow. Med. Image Anal. 16(3), 632–641 (2012).
Maier-Hein, L. et al. Surgical data science for next-generation interventions. Nat. Biomed. Eng. 1(9), 691–696 (2017).
Topol, E. J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 25(1), 44–56 (2019).
Senders, J. T. et al. An introduction and overview of machine learning in neurosurgical care. Acta Neurochir. 160(1), 29–38 (2018).
Gomez, E. D. et al. Objective assessment of robotic surgical skill using instrument contact vibrations. Surg. Endosc. 30(4), 1419–1431 (2016).
Guru, K. A. et al. Cognitive skills assessment during robot-assisted surgery: Separating the wheat from the chaff. BJU Int. 115(1), 166–174 (2015).
Vedula, S. S. et al. Task-level versus segment-level quantitative metrics for surgical skill assessment. J. Surg. Educ. 73(3), 482–489 (2016).
Lee, D. et al. Evaluation of surgical skills during robotic surgery by deep learning-based multiple surgical instrument tracking in training and actual operations. J. Clin. Med. 9(6), 1964 (2020).
Bamba, Y. et al. Automated recognition of objects and types of forceps in surgical images using deep learning. Sci. Rep. 11(1), 22571 (2021).
Khalid, S. et al. Evaluation of deep learning models for identifying surgical actions and measuring performance. JAMA Netw. Open 3(3), e201664–e201664 (2020).
Lavanchy, J. L. et al. Automation of surgical skill assessment using a three-stage machine learning algorithm. Sci. Rep. 11(1), 5197 (2021).
Lam, K. et al. Machine learning for technical skill assessment in surgery: A systematic review. NPJ Digit. Med. 5(1), 24 (2022).
Bissonnette, V. et al. Artificial intelligence distinguishes surgical training levels in a virtual reality spinal task. J. Bone Jt. Surg. Am. 101(23), e127 (2019).
Rosen, J. et al. Markov modeling of minimally invasive surgery based on tool/tissue interaction and force/torque signatures for evaluating surgical skills. IEEE Trans. Biomed. Eng. 48(5), 579–591 (2001).
Sugiyama, T. et al. Forces of tool-tissue interaction to assess surgical skill level. JAMA Surg. 153(3), 234–242 (2018).
Abiri, A. et al. Multi-modal haptic feedback for grip force reduction in robotic surgery. Sci. Rep. 9(1), 1–10 (2019).
Brown, J. D. et al. Using contact forces and robot arm accelerations to automatically rate surgeon skill at peg transfer. IEEE Trans. Biomed. Eng. 64(9), 2263–2275 (2016).
Tholey, G., Desai, J. P. & Castellanos, A. E. Force feedback plays a significant role in minimally invasive surgery: Results and analysis. Ann. Surg. 241(1), 102 (2005).
Wagner, C. R. et al. The benefit of force feedback in surgery: Examination of blunt dissection. Presence Tele Oper. Virtual Environ. 16(3), 252–262 (2007).
Zareinia, K. et al. A force-sensing bipolar forceps to quantify tool–tissue interaction forces in microsurgery. IEEE/ASME Trans. Mech. 21(5), 2365–2377 (2016).
Sutherland, G.R., et al., Bipolar Forceps with Force Measurement: (2021).
Sugiyama, T. et al. Tool-tissue interaction forces in brain arteriovenous malformation surgery. World Neurosurg. 102, 221–228 (2017).
Sutherland, G.R., et al., Machine Learning for Interconnected Surgical Theater Architecture, USPA, Editor (2021).
Albakr, A. et al. Introducing the nuances of Tool-tissue interaction forces in hemangioblastoma surgery. J. Neurol. Surg. Part B Skull. Base 83(01), 159 (2022).
Gan, L. S. et al. Quantification of forces during a neurosurgical procedure: A pilot study. World Neurosurg. 84(2), 537–548 (2015).
Sutherland, G.R., et al., Machine Learning-Based Surgical Instrument Characterization, USPA, Editor. (2021).
Huang, H., et al. Unet 3+: A full-scale connected unet for medical image segmentation. In: ICASSP 2020–2020 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). (IEEE, 2020).
Fawaz, H. I. et al. Inceptiontime: Finding alexnet for time series classification. Data Min. Knowl. Disc. 34(6), 1936–1962 (2020).
Buchlak, Q. D. et al. Machine learning applications to neuroimaging for glioma detection and classification: An artificial intelligence augmented systematic review. J. Clin. Neurosci. 89, 177–198 (2021).
Birkmeyer, J. D. et al. Surgical skill and complication rates after bariatric surgery. N. Engl. J. Med. 369(15), 1434–1442 (2013).
Baghdadi, A. et al. Data analytics interrogates robotic surgical performance using a microsurgery-specific haptic device. Expert Rev. Med. Devices 17(7), 721–730 (2020).
Hashimoto, D. A. et al. Artificial intelligence in surgery: Promises and perils. Ann. Surg. 268(1), 70 (2018).
Hung, A. J. et al. Utilizing machine learning and automated performance metrics to evaluate robot-assisted radical prostatectomy performance and predict outcomes. J. Endourol. 32(5), 438–444 (2018).
Jin, A., et al. Tool detection and operative skill assessment in surgical videos using region-based convolutional neural networks. In: 2018 IEEE Winter Conference on Applications of Computer Vision (WACV). (IEEE, 2018).
Forestier, G. et al. Surgical motion analysis using discriminative interpretable patterns. Artif. Intell. Med. 91, 3–11 (2018).
Lea, C. et al. Segmental spatiotemporal cnns for fine-grained action segmentation. In European Conference on Computer Vision (eds Leibe, B. et al.) (Springer, 2016).
Xue, B. et al. Use of machine learning to develop and evaluate models using preoperative and intraoperative data to identify risks of postoperative complications. JAMA Netw. Open 4(3), e212240–e212240 (2021).
Jiao, Y. et al. Continuous real-time prediction of surgical case duration using a modular artificial neural network. Br. J. Anaesth. 128(5), 829–837 (2022).
Funding
This study was supported by grants from (1) Alberta-Germany Collaborative Funds (German Canadian Centre for Innovation and Research); and (2) Canadian Institutes of Health Research (G. No. 390405)—Technology Commercialization. Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
A.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: A.B., Critical revision of the manuscript for important intellectual content: All authors. Machine learning analysis: A.B., Obtained funding: G.R.S., Administrative, operations, technical, or material support: S.L., Supervision: G. R.S. Additional appreciation: We extended our appreciation to all neurosurgery residents and the Division of Neurosurgery, Department of Clinical Neurosciences, Foothills Medical Center, Calgary AB, Canada.
Corresponding author
Ethics declarations
Competing interests
All authors are affiliated to the University of Calgary spin-off company called OrbSurgical Ltd., based in Calgary AB, Canada. The company was created to host new intellectual property (IP) arising from Project neuroArm-University of Calgary, which also includes IP related to the SmartForceps System.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed utablender a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baghdadi, A., Lama, S., Singh, R. et al. Tool-tissue force segmentation and pattern recognition for evaluating neurosurgical performance. Sci Rep 13, 9591 (2023). https://doi.org/10.1038/s41598-023-36702-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36702-3
This article is cited by
-
Tool-tissue interaction force in glioma surgery
Global Surgical Education - Journal of the Association for Surgical Education (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.