Abstract
Nausea often occurs in stressful situations, such as chemotherapy or surgery. Clinically relevant placebo effects in nausea have been demonstrated, but it remains unclear whether stress has an impact on these effects. The aim of this experimental study was to investigate the interplay between acute stress and placebo effects in nausea. 80 healthy female volunteers susceptible to motion sickness were randomly assigned to either the Maastricht Acute Stress Test or a non-stress control condition, and to either placebo treatment or no treatment. Nausea was induced by a virtual vection drum and behavioral, psychophysiological as well as humoral parameters were repeatedly assessed. Manipulation checks confirmed increased cortisol levels and negative emotions in the stressed groups. In the non-stressed groups, the placebo intervention improved nausea, symptoms of motion sickness, and gastric myoelectrical activity (normo-to-tachy (NTT) ratio). In the stressed groups, the beneficial effects of the placebo intervention on nausea and motion sickness remained unchanged, whereas no improvement of the gastric NTT ratio was observed. Results suggest that placebo effects on symptoms of nausea and motion sickness are resistant to experimentally-induced stress. Stress most likely interfered with the validity of the gastric NTT ratio to measure nausea and thus the gastric placebo effect.
Similar content being viewed by others
Introduction
Placebo effects occur not only after placebo interventions, but also contribute to the overall effect of almost any treatment1. A central goal of placebo research is therefore to better understand how contextual factors influence placebo effects. A little explored contextual factor in this regard is stress, which is ubiquitous in clinical settings.
According to the biopsychosocial model of arousal, stress integrates biological, psychological, and social factors2. Biological factors refer to the physiological responses during stress, such as the release of the stress hormones cortisol and adrenaline, which prepare the body to deal with the perceived threat. Psychological factors encompass emotional responses such as fear and anxiety, as well as cognitive appraisal of the stressful situation, which can further modify the stress response. Social factors refer to the social and environmental influences on the stress response such as interactions with others and social support from family and friends, which can buffer the effects of stress. Stress results from a dynamic interaction between these biological, psychological and social components2.
Placebo effects are likewise based on mutual interactions between biological, psychological and social factors. Research has shown, for example, that placebo interventions trigger physiological responses, such as the release of endogenous opioids, resulting in reduced pain perception3. Other studies have demonstrated placebo-induced changes in immune system functioning4 as well as cardiovascular and gastrointestinal responses5. Furthermore, psychological and cognitive dimensions play a central role in placebo effects. For example, if a person believes a sugar pill to be a powerful painkiller, the expectation of pain relief can lead to an actual reduction in pain intensity6. Social factors, including the patient's relationship with the healthcare provider and the level of social support, can further shape the psychological and biological placebo response7.
Given the complex interplay between biological, psychological and cognitive factors in both the stress response and the placebo effect, it is reasonable to suppose that both phenomena can interfere with each other. For example, findings from placebo research in the field of pain suggest that placebo interventions decrease experimental pain by reducing anticipatory stress and anxiety8,9. Furthermore, the presence of an additional stressor may further increase negative emotions, resulting in a smaller placebo effect on pain due to larger stress. Indeed, Lyby et al.10 induced acute fear in healthy volunteers by the anticipation of electrical shocks and demonstrated that the placebo effect on experimental pain was diminished by fear. However, a recent study by Roderigo et al.11 showed different results, as a stressful state induced by the Trier Social Stress Test enhanced placebo effects on urgency-to-defecate but did not affect visceral placebo hypoalgesia. The conflicting results may be due in part to different placebo effects and mechanisms for different symptoms and organ systems.5,12
In a series of studies, we recently investigated placebo effects in an experimental nausea paradigm and could show that a suitable placebo intervention combined with verbal suggestions significantly reduced nausea and motions sickness in healthy volunteers13,14. In addition, the placebo effect on nausea was associated with improved gastric activity in women as well as with meaningful changes of blood proteins15, indicating that the placebo effect in nausea can at least partially be objectively assessed. Our results were consistent with those of several studies demonstrating placebo effects on nausea and motion sickness in experimental and clinical settings13,14,16,17, although some studies have also shown negative or mixed results18,19,20. In patients, nausea often occurs in situations associated with high stress and apprehension per se, including medical interventions such as chemotherapy, radiotherapy, and surgical procedures21. Therefore, a better understanding of the interplay between acute stress and placebo effects in nausea could help to improve the management of this common, yet hard to treat symptom22.
In this study, we investigated for the first time the effects of negative emotions induced by acute experimental stress on placebo effects in nausea using an established placebo nausea paradigm13,14. Our aim was to induce significant stress, including the release of cortisol, as this may best reflect the intense stress of patients in a clinical setting. We elicited acute stress by the Maastricht Acute Stress Test (MAST), which is a validated stress protocol known to induce robust emotional and neuroendocrine responses; a validated control version of the test is also available23. Based on the original findings by Lyby et al. that acute fear reduced the effectiveness of placebo analgesia10, we hypothesized that a state of acute stress going along with negative emotions would diminish placebo effects on nausea and motion sickness, and, consequently, also the placebo-related improvement of the gastric correlate of nausea. We focused on female participants, as our previous placebo nausea study showed gastric placebo effects only in women14,15.
In addition to nausea, we explored for the first time how acute stress and the placebo intervention might influence time estimation in nausea. The rationale for including time estimation as an outcome was based on previous findings that time perception is modulated by acute stress and anxiety24,25 and by symptoms such as pain26.
Results
Participants
94 women participated in the study, of whom 12 women were assigned to real treatment (data not analysed), and two women dropped out on the testing day before randomization (1 withdrew consent, 1 developed circulatory problems during baseline measurement). Analyses were based on data from 80 participants assigned to placebo treatment (n = 41; 21 no stress, 20 stress) or no treatment (n = 39; 20 no stress, 19 stress). Participants were on average 24.3 years old (SD 3.2) and had received 17.6 years of formal education (SD 3.1). 95% were German native speakers; all participants were fluent in German. The majority of participants were Caucasian; 3 participants were of Asian descent. About two thirds (63%) of the participants used hormonal contraceptives. Sample characteristics at baseline are summarized in Table 1.
Nausea-related measures
After the placebo intervention, the expected severity of nausea, corrected for baseline levels, was significantly lower in the placebo groups compared with the control groups (analysis of covariance (ANCOVA) main effect of treatment, F(1,75) = 10.44, p = 0.002, ηp2 = 0.12; Table 2), thus confirming the induction of positive treatment expectations by the placebo intervention. Stress had no effect on nausea expectation (main effect of stress, F(1,75) = 0.05, p = 0.826, ηp2 = 0) and also did not interact with treatment (stress x treatment interaction, F(1,75) = 0.32, p = 0.575, ηp2 = 0).
The average intensity of nausea, corrected for baseline levels, was significantly lower in placebo-treated participants than in untreated participants (ANCOVA main effect of treatment, F(1,75) = 35.73, p < 0.001, ηp2 = 0.32; Table 2, Fig. 1A). Stress had no effect on nausea (main effect of stress, F(1,75) = 0.59, p = 0.445, ηp2 = 0.01) and did not interact with the placebo effect on nausea (stress x treatment interaction, F(1,75) = 0.967, p = 0.329, ηp2 = 0.01).
Mean effects (SE) of placebo treatment on (A) nausea ratinfigs, (B) motion sickness (SSMS), and (C) the gastric NTT-ratio in the stressed and the non-stressed groups. Note: *p < 0.05; ***p < 0.001 (Bonferroni-corrected post hoc tests). Abbreviations: SE, standard error; SSMS, Symptoms of Motion Sickness; NTT, normo-to-tachy.
Placebo treatment significantly reduced scores in the Subjective Symptoms of Motion Sickness questionnaire (SSMS, corrected for baseline levels; ANCOVA main effect of treatment, F(1,75) = 16.42, p < 0.001, ηp2 = 0.18; Table 2, Fig. 1B). Stress induction had no effect on motion sickness (main effect of stress F(1,75) = 1.81, p = 0.183, ηp2 = 0.02) and did not interact with the placebo effect on SSMS scores (stress x treatment interaction, F(1,75) = 0.003, p = 0.954, ηp2 = 0).
The ANCOVA for the gastric normo-to-tachy (NTT) ratio during the target period, corrected for baseline levels, showed a significant interaction between stress and treatment (F(1,75) = 4.68, p = 0.034, ηp2 = 0.06; Table 2, Fig. 1C). Bonferroni-corrected post hoc tests indicated a placebo effect in the non-stressed groups (p = 0.041), which was not observed in stressed groups (p = 0.323). Furthermore, stress tended to increase the gastric NTT ratio in untreated individuals (p = 0.056), but not in placebo-treated individuals (p = 0.287). Neither stress nor treatment had an independent effect on the gastric NTT ratio (main effect of stress, F(1,75) = 0.41, p = 0.525, ηp2 = 0.01; main effect of treatment, F(1,75) = 0.55, p = 0.462, ηp2 = 0.01).
Time estimation
The two-way analysis of variance (ANOVA) for time estimation after nausea revealed a significant stress x treatment interaction (F(1,74) = 8.35, p = 0.005, ηp2 = 0.1; Table 2). Bonferroni-corrected post hoc tests indicated a perception of faster passage of time after placebo administration in the non-stressed groups (p = 0.004) but not in the stressed groups (p = 0.258). At the same time, stress accelerated the subjective passage of time in the untreated groups (p = 0.009), but not in the placebo-treated groups (p = 0.165). Neither stress nor treatment had an independent effect on time estimation (main effect of stress, F(1,74) = 0.87, p = 0.354, ηp2 = 0.01; main effect of treatment, F(1,74) = 1.63, p = 0.205, ηp2 = 0.02).
Salivary cortisol and negative emotions
The mixed-design ANOVA for cortisol indicated a significant stress x time interaction (F(2.23, 162.39) = 17.46, p < 0.001, ηp2 = 0.19), which was due to significantly higher cortisol levels in the stressed groups than in non-stressed groups at all assessments after the MAST (Bonferroni-corrected p-values: p < 0.001, p < 0.001, p = 0.002, respectively; Table 3, Fig. 2A). In the stressed groups, cortisol levels were significantly higher after the MAST and before the onset of the vection stimulus compared with baseline values (Bonferroni-corrected p-values p = 0.002 and p = 0.001, respectively). In the non-stressed groups, cortisol levels declined during the experiment, with significantly lower levels after nausea and rest than during baseline (Bonferroni-corrected p-values < 0.001). The treatment x time interaction was not significant (F(2.23, 162.39) = 0.46, p = 0.652, ηp2 = 0.01), nor was the threefold interaction stress x time x treatment (F(2.23, 162.39) = 0.49, p = 0.630, ηp2 = 0.01).
Mean salivary cortisol levels (A) and mood ratings (B) at baseline, following the Maastricht Acute Stress Test (MAST), before nausea induction, after nausea induction, and at the end of the experiment. Note: **p < 0.01; ***p < 0.001 (Bonferroni-corrected post hoc tests for within-group comparisons); #p < 0.05; ##p < 0.01; ###p < 0.001, (Bonferroni-corrected post hoc tests for between-group comparisons);. Error bars indicate standard error. Abbreviations: NRS: Numeric rating scale.
The mixed-design ANOVA for mood ratings indicated a significant stress x time interaction (F(3.49, 261.56) = 8.57, p < 0.001, ηp2 = 0.1), which was due to significantly lower mood ratings in the stressed compared to the non-stressed groups after the MAST and before the onset of the vection stimulus (Bonferroni-corrected p < 0.001 and p = 0.018, respectively; Table 3, Fig. 2B). In the stressed groups, mood levels decreased from baseline to all follow-up measurements (all Bonferroni-corrected p-values < 0.001), while in the non-stressed groups, mood decreased significantly only from baseline to nausea and rest (Bonferroni-corrected p-values < 0.001). The treatment x time interaction was not significant (F(3.49, 261.56) = 0.86, p = 0.474, ηp2 = 0.01), nor was the interaction stress x time x treatment (F(3.49, 261.56) = 0.88, p = 0.468, ηp2 = 0.01).
The mixed-design ANOVA for state anxiety indicated a significant stress x time interaction (F(1.85,138.9) = 24.57, p < 0.001, ηp2 = 0.25), which was due to higher state anxiety levels after the MAST in the stressed compared to the non-stressed participants (Bonferroni-corrected p < 0.001; Table 3). In the stressed participants, state anxiety increased from baseline to MAST (Bonferroni-corrected p < 0.001) and rest (Bonferroni-corrected p = 0.014). In the non-stressed participants, state anxiety increased from baseline to rest (Bonferroni-corrected p = 0.002). The treatment x time interaction was not significant (F(1.85,138.9) = 0.75, p = 0.467, ηp2 = 0.01), nor was the threefold interaction stress x time x treatment (F(1.85,138.9) = 0.84, p = 0.426, ηp2 = 0.01).
The mixed-design ANOVA for ratings of inner tension indicated a significant stress x time interaction (F(3.35, 247.77) = 3.32, p = 0.017, ηp2 = 0.04), with significantly higher ratings of inner tension after the MAST in the stressed compared to the non-stressed groups (Bonferroni-corrected p = 0.01; Table 3). In the stressed groups, inner tension increased from baseline to MAST, nausea, and rest (Bonferroni-corrected p-values, p < 0.001, p < 0.001, p = 0.002, respectively). In the non-stressed groups, inner tension increased from baseline to nausea and rest (Bonferroni-corrected p < 0.001). In addition, a significant treatment x time interaction was observed (F(3.35, 247.77) = 3.77, ηp2 = 0.05), with significantly lower tension ratings after nausea induction in placebo-treated participants compared with untreated ones (Bonferroni-corrected p = 0.009; Table 3). The threefold interaction stress x time x treatment was not significant (F(3.35, 247.77, ηp2 = 0.02) = 1.09, p = 0.357).
Explorative correlations
To better understand the relationships between the gastric NTT ratio and nausea, motion sickness, stress, negative emotions, and time estimation, explorative correlation analyses of parameter changes from baseline to nausea were performed for the stressed and the non-stressed groups (Table 4). In the non-stressed groups, the gastric NTT ratio correlated negatively with nausea (r = -0.35, p = 0.025), whereas in the stressed groups, a positive association between the gastric NTT ratio and ratings of inner tension emerged (r = 0.33, p = 0.042).
Discussion
This is the first study to investigate the interplay between acute stress and placebo effects in nausea. In line with previous studies13,14,16, the expectancy manipulation induced a strong placebo effect on the severity of nausea and motion sickness in the non-stressed groups. We also replicated our recent finding of a placebo effect on the gastric NTT-ratio in a female population in the absence of experimental stress15. The novel finding of the present study is that in the stressed groups, the placebo effect on nausea and motion sickness remained unaffected, while a placebo effect on the gastric NTT ratio was no longer observed.
Using a visceral pain model, Roderigo et al.11 recently demonstrated that acute stress had no impact on placebo analgesia, but enhanced the placebo effect on urgency-to-defecate. Our results partially confirm these results, as acute stress did not affect the placebo effect at the level of symptoms, i.e., nausea and motion sickness. Thus, placebo effects on symptom perception, evaluation and reporting appear to be robust against acute stress. However, our results suggest that gastric myoelectric activity responded differently to placebo treatment depending on whether acute stress was induced or not. Only in the non-stressed participants, and consistent with previous studies27,28, a negative association between inner-tension ratings and the gastric NTT ratio emerged, indicating that larger stress was associated with less normal gastric activity. In the stressed groups, a positive association between inner-tension ratings and the gastric NTT ratio was found, suggesting that larger stress was associated with increased normal gastric activity. Several studies indicate that laboratory stressors such as shock avoidance, speech preparation, hand grip, cold stress, audio stress, Stroop task, and arithmetic tasks decrease the spectral power in the normogastric frequency band and/or increase it in the tachygastric frequency band29,30,31,32, resulting in a reduced NTT ratio. Other studies, however, reported different results and concluded that the direction of changes to acute stress may depend on the fasting state of the participants: after a meal, when normal gastric motility is high, the inhibitory effects of stress on gastric myoelectrical activity are usually large and significant, while in the fasting state, when gastric motility is low, the effects of stress on gastric myoelectrical activity may be absent, or even go in the opposite direction33,34. Since our experiments were performed in the fasting state, stress may have induced a paradoxical increase of normal gastric motility, thereby interfering with the validity of the gastric NTT ratio as a measure of nausea.
The question of whether placebo-induced expectations can affect peripheral physiological systems remains controversial. Although autonomic changes can be reliably induced by placebo interventions35,36, it is unclear whether these changes form an intrinsic part of the neurobiological placebo response or represent autonomic correlates of symptoms such as pain and nausea, which are expected to decrease when symptoms improve5. Furthermore, in a clinical trial of asthma patients, a placebo inhaler was found to improve asthma symptoms while lung function did not change (FEV1)37, suggesting that beyond autonomic correlates, placebo interventions may have no effects on pathophysiological processes. With this in mind, it would be interesting to investigate additional, more sensitive physiological parameters to elucidate the nature of peripheral placebo responses. In a recent study, for example, we used proteomics analyses of peripheral blood samples to identify protein signatures associated with the placebo effect in nausea15. Among other changes, placebo effects were related to alterations in micro-inflammatory proteins and neuropeptides such as neurexin. Various biological pathways were found to be involved, including the acute-phase response as well as regulation of grooming behavior15. Further studies of this type could help to clarify the extent to which peripheral changes are part of the neurobiological placebo response or rather consequences of reduced stress and autonomic arousal.
In an exploratory approach, we used time estimation as a novel and promising marker to monitor negative emotional states25. For this, we asked participants to retrospectively rate how fast the period of nausea induction had passed for them. Several studies indicate that acute experimental stress24, high levels of anxiety in patients just before they had to undergo chemotherapy25, and states of high arousal in everyday life38 are associated with a subjective acceleration of time, whilst low-to-moderate arousal levels in everyday life38 as well as the unpleasant experience of pain26 and waiting39 are associated with a subjective “dragging” of time. Our results are consistent with these findings in the sense that acute stress accelerated the passage of time in the untreated groups, just as placebo treatment accelerated the passage of time, but only in the non-stress condition, and presumably by reducing the unpleasantness of nausea. Similar to the findings on gastric myoelectric activity, acute stress may have masked the effects of the placebo intervention on time estimation by inducing effects in the same direction.
This is the first study to investigate the influence of acute stress on subjective and physiological placebo effects in nausea. We applied a randomized controlled, partially blinded design with behavioral, psychophysiological and humoral outcome variables and obtained a high internal validity of the results. Manipulation checks indicated successful induction of acute stress and positive expectations in the respective groups. Nonetheless, the study has several limitations. The relatively homogenous, female-only, young and healthy study population reduces the generalizability of the results. At the same time, variance in physiological placebo effects associated with sex15,40 could this way be removed, rendering the results for women more conclusive. Furthermore, even though the overall sample size was considerably large, subgroups were limited in size and small effects could have been missed due to the lack of statistical power. In addition, we focused on nausea induced by a virtual vection drum, which is a well-established experimental model of nausea induction41, and we pre-selected participants susceptible to motion sickness. Even though a positive history of motion sickness increases the risk to experience nausea also in clinical settings42, the generalizability of our results to other types of nausea, like chemotherapy-induced nausea and postoperative nausea and vomiting, remains to be investigated. Furthermore, the choice of the EGG to measure gastric changes to both placebo and stress interventions can be criticized, as it is knowingly influenced by both, stress and nausea. However, EGG is so far the most promising method for noninvasively measuring gastric correlates of visually-induced nausea27 as well as associated placebo effects15. Finally, it should be mentioned that the study protocol was retrospectively registered in a public repository. Preregistration has meanwhile been identified as one of the factors associated with increased reproducibility and reliability of empirical studies43.
In summary, our results suggest that acute stress did not alter placebo effects on nausea and motion sickness. At the same time, stress appeared to interfere with the validity of the gastric NTT ratio to measure nausea and thus the gastric placebo effect, possibly by increasing normal gastric motility in the fasting state. Future studies should investigate whether placebo effects on the gastric NTT ratio can also be induced in the fed state and how acute stress alters placebo effects in such situations. In addition, the interplay between stress and placebo effects in nausea should also be investigated in healthy men as well as in patients, who are usually exposed to a variety of internal and external stressors.
Methods
Study design
We performed a randomized controlled study in healthy female volunteers who were assigned to undergo either a stress or a control task and, after completion, were treated with either a placebo treatment or remained untreated. A small active treatment group (n = 12) was also included to permit blinded administration of placebo treatment (data not analyzed). The study was conducted at the Institute of Medical Psychology, Ludwig Maximilian University (LMU) of Munich in accordance with all relevant ethical guidelines and regulations for human participants. The study protocol was approved by the ethics committee of the Medical Faculty at LMU Munich (# 401–13) and written informed consent was obtained from all participants. The study was registered at the German Clinical Trials Register (DRKS; 08/12/2022, DRKS00027033).
Participants
Healthy female participants (18–40 years old) were recruited using flyers in Munich universities and postings on social media. We included only women to reduce heterogeneity, as women and men have been found to respond differently to nausea44. Further, a placebo effect on nausea-related gastric myoelectrical activity could only be shown in women in our recent study15. Interested volunteers were screened for their susceptibility to motion sickness using the Motion Sickness Susceptibility Questionnaire (MSSQ)45. If the MSSQ indicated moderate to severe, but not extreme motion sickness susceptibility (score 80–200), participants were further screened for eligibility in a structured telephone interview. Predefined exclusion criteria comprised contraindications for the use of transcutaneous electrical nerve stimulation (TENS; e.g., implanted devices or metal implants, coagulopathies and hypercoagulable states including surgery in the past 4 weeks), history of inner ear disease (e.g., Morbus Ménière, acute hearing loss), acute or chronic somatic and/or psychiatric conditions (i.e. cardiovascular disease, epilepsy, cancer, substance abuse, skin disease), regular use of medications (except for hormonal contraception, L-thyroxine and allergic rhinitis medications), and current pregnancy or breastfeeding. Volunteers with clinically relevant scores on the Hospital Anxiety and Depression Scale (HADS)46 were also excluded, using a cut-off point of 8 for anxiety and depression, respectively47. Volunteers who met all inclusion criteria and none of the exclusion criteria were invited to a 20-min screening session in the laboratory to test their response to the nauseogenic vection stimulus. Participants who developed at least moderate nausea (5 on a numeric rating scale (NRS) from 0 to 10) for at least 3 min and did not experience extreme nausea (> 9) or vomiting at any point were included in the study.
Study procedure
To minimise habituation effects to the vection stimulus, the experimental session was scheduled no earlier than 48 h after the screening session. For participants who did not use hormonal contraception, the experiment was conducted during the luteal phase of the menstrual cycle to minimise the effects of gonadal hormones on symptoms, gastric activity, and stress responses48. To account for the circadian variability of cortisol levels, all sessions took place between noon and 6 pm49. Participants were instructed not to eat or drink anything in the 3 h before the experiment. To ensure the participants’ fasting state, blood glucose levels were determined upon arrival from finger blood samples using a BG Star device (Sanofi-Aventis, Hannover, Germany). Participants were seated in a comfortable chair and asked to complete several questionnaires. The respiration belt and the skin electrodes were attached, physiological measurements were started, and the first salivary cortisol sample was collected.
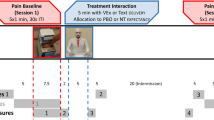
The experimental procedure is shown in Fig. 3. After a 10-min resting period during which baseline measurements were taken, participants were randomly allocated to either the stress or the control task, and the respective task was performed. Thereafter, participants were randomly assigned to treatment (active treatment or placebo/sham treatment; only placebo group analysed as above) or no treatment. In treatment groups, the treatment was initiated for 20 min. After the first 10 min of the treatment period, the vection stimulus was applied for 20 min. The experimental session ended with a 10-min resting period. The target period for the statistical analyses comprised the second 10-min period of the vection stimulus, when the treatment had already ended.
Treatments, randomization and blinding
Participants in the treatment groups received a standardized positive verbal suggestion of nausea improvement (for details, see Aichner, et al. 14). Then, a programmable TENS device (Digital EMS/TENS unit SEM 42, Sanitas, Uttenweiler, Germany) was applied. In the placebo treatment groups, the TENS electrodes were attached proximal and distal to a dummy acupuncture point located on the ulnar side of both forearms 50, and the superficial massage program of the TENS device was turned on for 20 min to induce a slight tingling sensation at the electrode site14. For the real TENS intervention in the active treatment group, the electrodes were placed around ‘PC6’, a validated acupuncture point for the treatment of nausea51, and the TENS program was turned on for 20 min. Participants in the control groups did not receive any treatment. They were informed about the importance of untreated groups and were asked at the end of the experiment to rate their degree of disappointment about allocation to the control group. On an NRS from 0 (not at all disappointed) to 10 (extremely disappointed), disappointment was rated low at 2.05 on average (median 1, range 0–7), which makes it unlikely that it affected the results.
The recruited women consecutively received a participant number from a list. Before the start of the study, an independent researcher assigned all participant numbers in advance to the respective experimental conditions using the random sequence generator implemented in Microsoft Excel. For each participant number, the independent researcher prepared two sealed envelopes with respective group assignment information. The first envelope assigned participant numbers to the “stress” or “non-stress” condition and was opened by the person conducting these tasks. After completion of the task, the main experimenter entered the room and opened the second envelope containing the treatment assignment information, thus remaining blinded regarding the stress or non-stress condition. Participants in the treatment groups were blinded regarding the type of treatment (placebo or active treatment), whereas participants in the no treatment control group were necessarily unblinded.
Nausea induction
Nausea was induced by a vection stimulus generated by a virtual optokinetic drum13,14. Vection stimuli create the illusion of self-movement which causes visually-induced motion sickness (VIMS) in susceptible participants52. VIMS is frequently used to induce nausea in experimental settings as it avoids pharmacological agents and allows participants to be stationary, which facilitates data acquisition53. We induced nausea for 20 min according to an established protocol13,14. Black and white stripes moving from left to right with a speed of 60 degrees/second were projected on a semicylindrical semitransparent screen surrounding the participant. The distance between the screen and the participants’ eyes was 30 cm. This way, the projection covered the entire visual field of participants. Participants were advised to look at the screen without fixating on single stripes.
Stress induction
Stress was induced using the MAST23. The MAST combines elements of the cold pressure test54 with social evaluation in a mental arithmetic task. Participants in the non-stressed groups underwent a control version of the stress test, involving hand-immersion in warm water and counting instead of performing calculations23. Both tests were performed by two persons who were not further involved in the experiment. To participants, the stress test or its control version was described as a “task which may include unpleasant stimuli”.
Outcome measures and manipulation checks
Nausea intensity was assessed using an 11-point NRS with the poles “not nauseated at all” (0) and “imminently vomiting” (10). To account for the wave-like character of nausea22, ratings were obtained every minute during the 20-min nausea induction period and averaged for every 10 min. Motion sickness severity was assessed by the SSMS (adapted from Graybiel, et al. 55), with scores of 0 to 3 assigned to responses of none, slight, moderate, and severe symptoms of dizziness, headache, nausea/urge to vomit, tiredness, sweating, and stomach awareness, respectively14. The EGG served to examine changes in gastric myoelectrical activity related to nausea (see below). The expected maximum intensity of nausea during the experiment was assessed using an 11-point NRS with poles of “no nausea “ (0) and “imminently vomiting” (10).
Salivary cortisol, mood ratings (11-point NRS from “worst mood” (0) to “best mood ever” (10)56), ratings of inner tension (11-point NRS from “no inner tension” to “extreme inner tension”), and state anxiety (State-Trait-Anxiety Inventory; STAI57) were used to check the success of the stress task and to examine possible interactions with placebo treatment.
In an exploratory approach, we also assessed time estimation using a 10 cm long visual analogue scale (VAS), asking participants to rate how fast time had passed for them during the 20-min time period of nausea induction. Answers were given by a vertical stroke ranging somewhere between the endpoints of “extremely slowly” (0) to “extremely fast” (10)39.
Physiological measurements
EGG data were recorded with BIOPAC MP 150 device (BIOPAC Systems, Goleta, CA, USA) and AcqKnowledge 4.1 software for data acquisition. We followed the same analysis protocol as in our previous study15. In short, after processing the raw data, frequency spectrums during baseline and during the target period were analysed with Fourier transformation and the NTT ratio was calculated as the proportion of mean spectral values of the normogastric frequency band (2.5–3 Hz) and the tachygastric frequency band (3.75–9.75 Hz)19. Lower NTT ratios are associated with higher nausea58,59. In addition to the EGG, respiratory activity (to control the EGG for respiratory artifacts), and electrocardiogram (results not reported here) were recorded with the BIOPAC MP 150 system.
Salivary cortisol levels were obtained with Salivette Cortisol® swabs (Sarstedt, Germany) at various time points as detailed in Fig. 3. Samples were stored on ice during the session, then centrifuged for 3 min with 3000 rpm at 4 °C and stored at -20 °C. Samples were analysed in duplicate using the IBL International Cortisol Saliva ELISA Kit (catalogue number RE52611) following the manufacturer’s protocol. The sensitivity of the cortisol assay was 0.04 ng/ml and inter- and intra-assay coefficients of variance were < 10%. Individual cortisol values were transformed to logarithmic values with a constant score of 4 added to obtain positive values.
Statistical analyses
Statistical analyses were performed with SPSS statistics software (version 26, IBM). To address the primary hypothesis testing an interaction of stress and treatment, average nausea ratings during the target period (i.e., during the second half of nausea stimulation; Fig. 3) were subjected to a two-way ANCOVA), with “stress” (yes/no) and “treatment” (placebo treatment/no treatment) as between-subject factors and nausea levels at baseline included as a covariate. Assuming a large effect size partial eta-squared of 0.15 for interaction effects in the analysis of variance, we estimated a priori that 20 subjects per group would be needed to give 95% power to detect a significant difference (with a type 1 error of 5%; calculated by MorePower Version 6.0). Gastric NTT ratio during the second half of nausea stimulation as well as SSMS scores at the end of nausea induction were subjected to two-way ANCOVAs, with the between-subject factors “stress” (yes/no) and “treatment” (placebo/no treatment) and baseline levels included as covariates. Expectation ratings after expectancy manipulation were compared between placebo groups and control groups using a baseline-adjusted two-way ANCOVA with the between-subject factors “stress” (yes/no) and “treatment” (placebo/no treatment). The effect of the MAST on salivary cortisol, mood and inner-tension ratings was evaluated using mixed-design ANOVAs with the within-subject factor “time” (baseline, after the MAST, before nausea stimulation), and the between-subject factor “stress” (yes/no). Greenhouse–Geisser corrected results are reported when Mauchly's test indicated violation of sphericity. Effect sizes are reported as partial eta squared (ηp2). Results were considered statistically significant if p < 0.05 (two-sided).
Data availability
The raw data are available from the corresponding author upon request.
References
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: Minimize, maximize or personalize? Nat. Rev. Drug Discov. 12, 191–204 (2013).
Blascovich, J. & Tomaka, J. The biopsychosocial model of arousal regulation. In: Advances in Experimental Social Psychology Vol. 28, 1–51 (Elsevier, 1996).
Meissner, K. et al. The placebo effect: advances from different methodological approaches. J. Neurosci. 31, 16117–16124 (2011).
Hadamitzky, M., Sondermann, W., Benson, S. & Schedlowski, M. Placebo effects in the immune system. Int. Rev. Neurobiol. 138, 39–59 (2018).
Meissner, K. Placebo responses on cardiovascular, gastrointestinal, and respiratory organ functions. Handb. Exp. Pharmacol. 225, 183–203 (2014).
Colloca, L. The placebo effect in pain therapies. Annu. Rev. Pharmacol. Toxicol. 59, 191–211 (2019).
Evers, A. W. et al. Implications of placebo and nocebo effects for clinical practice: expert consensus. Psychother. Psychosom. 87, 204–210 (2018).
Aslaksen, P. M., Bystad, M., Vambheim, S. M. & Flaten, M. A. Gender differences in placebo analgesia: Event-related potentials and emotional modulation. Psychosom. Med. 73, 193–199 (2011).
Aslaksen, P. M. & Flaten, M. A. The roles of physiological and subjective stress in the effectiveness of a placebo on experimentally induced pain. Psychosom. Med. 70, 811–818 (2008).
Lyby, P. S., Forsberg, J. T., Asli, O. & Flaten, M. A. Induced fear reduces the effectiveness of a placebo intervention on pain. Pain 153, 1114–1121 (2012).
Roderigo, T. et al. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain 158, 1489–1498 (2017).
Wager, T. D. & Atlas, L. Y. The neuroscience of placebo effects: Connecting context, learning and health. Nat. Rev. Neurosci. 16, 403–418 (2015).
Müller, V., Remus, K., Hoffmann, V., Tschöp, M. H. & Meissner, K. Effectiveness of a placebo intervention on visually induced nausea in women—A randomized controlled pilot study. J. Psychosom. Res. 91, 9–11 (2016).
Aichner, S. et al. The role of tactile stimulation for expectation, perceived treatment assignment and the placebo effect in an experimental nausea paradigm. Front. Neurosci. 13, 1212 (2019).
Meissner, K. et al. Molecular classification of the placebo effect in nausea. PLoS ONE 15, e0238533 (2020).
Horing, B. et al. Reduction of motion sickness with an enhanced placebo instruction: An experimental study with healthy participants. Psychosom. Med. 75, 497–504 (2013).
Quinn, V. F. & Colagiuri, B. Sources of placebo-induced relief from Nausea: The role of instruction and conditioning. Psychosom. Med. 78, 365–372 (2016).
Levine, M. E., Stern, R. M. & Koch, K. L. The effects of manipulating expectations through placebo and nocebo administration on gastric tachyarrhythmia and motion-induced nausea. Psychosom. Med. 68, 478–486 (2006).
Weimer, K., Horing, B., Muth, E. R. & Enck, P. How to study placebo responses in motion sickness with a rotation chair paradigm in healthy participants. J. Vis. Exp. 94, e52471(2014).
Barnes, K., Yu, A., Josupeit, J. & Colagiuri, B. Deceptive but not open label placebos attenuate motion-induced nausea. J. Psychosom. Res. 125, 109808 (2019).
Holmes, A. M., Rudd, J. A., Tattersall, F. D., Aziz, Q. & Andrews, P. L. Opportunities for the replacement of animals in the study of nausea and vomiting. Br. J. Pharmacol. 157, 865–880 (2009).
Andrews, P. L. & Sanger, G. J. Nausea and the quest for the perfect anti-emetic. Eur. J. Pharmacol. 722, 108–121 (2014).
Smeets, T. et al. Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 37, 1998–2008 (2012).
Bagley, S. L., Massner, K., Schneider, C., Miller, A. & Moore, K. That moment felt like forever: Stress effects on time perception in males. Timing Time Percept. 1, 1–16 (2021).
Donev, I. S., Ivanova, M. S. & Conev, N. V. Fast time perception is associated with high levels of anxiety in cancer patients prior to starting chemotherapy. Biosci. Trends 14, 35–41 (2020).
Wing, J. Investigating the interaction between pain intensity and time perception and whether anxiety is a moderating factor on this relationship. Manchester Metropolitan University Psychology Journal (Dissertations) (2013).
Hu, S., Stern, R. M., Vasey, M. W. & Koch, K. L. Motion sickness and gastric myoelectric activity as a function of speed of rotation of a circular vection drum. Aviat. Space Environ. Med. 60, 411–414 (1989).
Stern, R. The psychophysiology of nausea. Acta Biol. Hung. 53, 589–599 (2002).
Muth, E. R., Koch, K. L., Stern, R. M. & Thayer, J. F. Effect of autonomic nervous system manipulations on gastric myoelectrical activity and emotional responses in healthy human subjects. Psychosom. Med. 61, 297–303 (1999).
Gianaros, P. J., Quigley, K. S., Mordkoff, J. T. & Stern, R. M. Gastric myoelectrical and autonomic cardiac reactivity to laboratory stressors. Psychophysiology 38, 642–652 (2001).
Kim, N., Seo, W., Kim, S. & Park, S.-M. Electrogastrogram: Demonstrating feasibility in mental stress assessment using sensor fusion. IEEE Sens. J. 21, 14503–14514 (2020).
Lei, Y. & Chen, J. Inhibitory effects of various types of stress on gastric tone and gastric myoelectrical activity in dogs. Scand. J. Gastroenterol. 44, 557–563 (2009).
Stern, R. M., Vasey, M. W., Hu, S. & Koch, K. L. Effects of cold stress on gastric myoelectric activity. Neurogastroenterol. Motil. 3, 225–228 (1991).
Yin, J., Levanon, D. & Chen, J. Inhibitory effects of stress on postprandial gastric myoelectrical activity and vagal tone in healthy subjects. Neurogastroenterol. Motil. 16, 737–744 (2004).
Meissner, K. The placebo effect and the autonomic nervous system: Evidence for an intimate relationship. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 366, 1808–1817 (2011).
Meissner, K., Distel, H. & Mitzdorf, U. Evidence for placebo effects on physical but not on biochemical outcome parameters: A review of clinical trials. BMC Med. 5, 1–11 (2007).
Wechsler, M. E. et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N. Engl. J. Med. 365, 119–126 (2011).
Droit-Volet, S. & Wearden, J. H. Experience Sampling Methodology reveals similarities in the experience of passage of time in young and elderly adults. Acta Psychol. (Amst) 156, 77–82 (2015).
Jokic, T., Zakay, D. & Wittmann, M. Individual differences in self-rated impulsivity modulate the estimation of time in a real waiting situation. Timing Time Percept. 6, 71–89 (2018).
Haile, A. et al. Central correlates of placebo effects in nausea differ between men and women. Brain Behav. 12, e2685 (2022).
Kennedy, R. S., Drexler, J. & Kennedy, R. C. Research in visually induced motion sickness. Appl. Ergon. 41, 494–503 (2010).
Meissner, K. et al. Individual factors contributing to nausea in first-time chemotherapy patients: A prospective cohort study. Front. Pharmacol. 10, 410 (2019).
Segerstrom, S. C., Diefenbach, M. A., Hamilton, K., O'Connor, D. B. & Tomiyama, J. A. Open science in health psychology and behavioral medicine: A statement from the Behavioral Medicine Research Council. Health Psychology (2023).
Meissner, K., Enck, P., Muth, E. R., Kellermann, S. & Klosterhalfen, S. Cortisol levels predict motion sickness tolerance in women but not in men. Physiol. Behav. 97, 102–106 (2009).
Golding, J. F. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res. Bull. 47, 507–516 (1998).
Zigmond, A. S. & Snaith, R. P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 67, 361–370 (1983).
Bjelland, I., Dahl, A. A., Haug, T. T. & Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 52, 69–77 (2002).
Kirschbaum, C., Kudielka, B. M., Gaab, J., Schommer, N. C. & Hellhammer, D. H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med 61, 154–162 (1999).
Dickmeis, T., Weger, B. D. & Weger, M. The circadian clock and glucocorticoids–interactions across many time scales. Mol. Cell. Endocrinol. 380, 2–15 (2013).
Witt, C. M. et al. Stimulation of gastric slow waves with manual acupuncture at acupuncture points ST36 and PC6–a randomized single blind controlled trial. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 24(438–445), e211–432 (2012).
Arnberger, M. et al. Monitoring of meeting abstracts at the P6 acupuncture point reduces the incidence of postoperative nausea and vomiting. J. Am. Soc. Anesthesiol. 107, 903–908 (2007).
Hettinger, L. J. & Riccio, G. E. Visually induced motion sickness in virtual environments. Teleoperators Virtual Environ. 1, 306–310 (1992).
Napadow, V. et al. The brain circuitry underlying the temporal evolution of nausea in humans. Cereb. Cortex 23, 806–813 (2013).
Lovallo, W. The cold pressor test and autonomic function: A review and integration. Psychophysiology 12, 268–282 (1975).
Graybiel, A., Wood, C., Miller, E. & Cramer, D. Diagnostic criteria for grading the severity of acute motion sickness. Aerosp. Med. 39, 453–455 (1968).
Ferrucci, R. et al. Cerebellum and processing of negative facial emotions: Cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn. Emot. 26, 786–799 (2012).
Spielberger, C. State-Trait Anxiety Inventory (Wiley, 2010).
Stern, R. M., Koch, K. L., Stewart, W. R. & Lindblad, I. M. Spectral analysis of tachygastria recorded during motion sickness. Gastroenterology 92, 92–97 (1987).
Farmer, A. D. et al. Visually induced nausea causes characteristic changes in cerebral, autonomic and endocrine function in humans. J. Physiol. 593, 1183–1196 (2015).
Funding
This research was supported by the German Research Foundation (DFG, FOR 1328, ME-3675/1-1). Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
K.M. and M.H.T. conceived the experiment, C.J., E.O., A.H., V.H., B.J., L.S., and M.L. conducted the experiment, C.J. and K.M. analysed the results, C.J. and K.M. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacob, C., Olliges, E., Haile, A. et al. Placebo effects on nausea and motion sickness are resistant to experimentally-induced stress. Sci Rep 13, 9908 (2023). https://doi.org/10.1038/s41598-023-36296-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-36296-w
This article is cited by
-
Exploring neurophysiological correlates of visually induced motion sickness using electroencephalography (EEG)
Experimental Brain Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.