Abstract
The hippocampus is affected early in Alzheimer’s disease (AD) and altered hippocampal functioning influences normal cognitive aging. Here, we used task-based functional MRI to assess if the APOE ɛ4 allele or a polygenic risk score (PRS) for AD was linked to longitudinal changes in memory-related hippocampal activation in normal aging (baseline age 50–95, n = 292; n = 182 at 4 years follow-up, subsequently non-demented for at least 2 years). Mixed-models were used to predict level and change in hippocampal activation by APOE ɛ4 status and PRS based on gene variants previously linked to AD at p ≤ 1, p < 0.05, or p < 5e−8 (excluding APOE). APOE ɛ4 and PRSp<5e−8 significantly predicted AD risk in a larger sample from the same study population (n = 1542), while PRSp≤1 predicted memory decline. APOE ɛ4 was linked to decreased hippocampal activation over time, with the most prominent effect in the posterior hippocampi, while PRS was unrelated to hippocampal activation at all p-thresholds. These results suggests a link for APOE ɛ4, but not for AD genetics in general, on functional changes of the hippocampi in normal aging.
Similar content being viewed by others
Introduction
The biological processes and genetic factors underlying the pathology behind Alzheimer’s disease (AD) and neurocognitive changes in the range of normal aging are partly overlapping1,2. Given that the strongest risk factor for AD is increased age, and that early symptoms of AD are difficult to discriminate from aging1, it needs to be elucidated how individual variation in normal neurocognitive aging relates to the progression of AD.
AD is considered to have oligogenetic heritability patterns, where the apolipoprotein E (APOE) ε4 allele constitutes the single strongest genetic risk factor for the disease3. The role of APOE in the etiology of AD is not fully understood. Previous studies have linked APOE to AD-related mechanisms, including the pathophysiology behind amyloid-β plaques, tau neurofibrils, and neuroinflammation3. Recent studies also suggest that APOE ε4 may be important for normal cognitive aging independent from AD pathologies4.
Several other genetic variants have been associated with AD with weaker effect sizes, linked to genes that are involved in lipid metabolism-, amyloid-β-, tau- and immune pathways5,6. Polygenic risk scores (PRS) for AD have been used to study the additive effect of multiple gene variants across the whole genome on endophenotypes and to predict disease onset7. We have recently shown that both APOE ε4 and PRS for AD predicted aging-related cognitive decline across 25 years in individuals that remained healthy at follow-up, where the effect of PRS was seen already six years prior to diagnostic follow-up8.
The hippocampus, an important brain area for memory functioning, is structurally and functionally linked to both cognitive aging and development of AD. Atrophy of the hippocampus is observed already at preclinical stages of AD9. Both APOE ε4 and AD PRS have been linked to hippocampal volume in healthy individuals10,11, although a larger study found the effect of AD PRS to be attenuated when removing the APOE locus from the PRS12. Longitudinal changes in hippocampal volume have been linked to episodic memory decline in APOE ε4 carriers but not in non-carriers13. fMRI studies of hippocampal activation during memory tasks have shown both hypo- 14,15,16 and hyper-activation17,18,19,20,21,22, i.e. lower or higher amplitude of the fMRI signal, in individuals with mild cognitive impairment (MCI) or AD compared to controls. Altered hippocampal activation has also been reported in cross-sectional studies of healthy APOE ε4 allele carriers relative to non-carriers23,24,25,26,27,28. In addition, altered hippocampal activation has been associated with high AD PRS29,30.
The aim of the present study was to examine if APOE ε4 or AD PRS is associated with level and age-related change in hippocampal activation in healthy individuals. In a previous study using the same data, we observed longitudinal decrease in activation of the anterior hippocampi during memory encoding in aging31. Based on this finding, we additionally examined whether a possible association would differ between anterior and posterior hippocampal regions. Our primary hypothesis was that genetic risk factors for AD would magnify the decreases in hippocampal activity previously associated with normal aging.
Methods
Participants
Study participants belong to the longitudinal population-based prospective Betula cohort study on memory, health and aging. For an extensive description of the whole Betula project, please see our review by Nyberg et al.2. Participants in the Betula study were randomly selected from the population registry in Umeå, Sweden. The aim of the Betula study was to explore memory functioning in adulthood and late life, and to explore early signs and risk factors of dementia. In the Betula project, extensive cognitive data as well as various other variables have been repeatedly assessed 4–5 years apart at six different timepoints (wave 1–6), where brain imaging data is available for wave 5 and wave 6. As previously reported by Nyberg et al.2, the memory performance of individuals that underwent fMRI was on average 0.19 standard deviations above that of the total sample of the Betula study, and this difference was more pronounced in older individuals driven by selective attrition before wave 5. Exclusion criteria for the brain scanning sessions were contraindications to MRI or notable artifacts in the fMRI acquisition, history of known neurologic or psychiatric disease, or dementia diagnosis. Individuals who developed dementia until the last diagnostic screening performed in Betula (2015–2017) were excluded from analysis of brain activation (n = 14). A total number of 292 subsequently healthy individuals (141 males and 151 females) aged 50–95 years at the first scanning session (2009–2010) were included, of which 182 returned for a second fMRI scan four years later (2013–2014). To evaluate whether APOE ɛ4 carriage and/or a PRS predicts the risk of developing AD, we included a larger sample from the Betula project2, of which the brain imaging sample in this study constitutes a subset. PRSs were available for n = 1746 subjects. Subjects with missing data for age and sex (n = 33), unknown dementia diagnosis (n = 116) or with a dementia diagnosis at baseline or before baseline (n = 55) were excluded. Participants with the confounding APOE ε2/APOE ε4 genotype)46 were not included (n = 40). The final sample included 1542 individuals. The study protocol of this project was approved by the local ethics board at Umeå University (Regionala etikprövningsnämnden Umeå, Sweden) and the protocol was followed throughout the study period. All participants provided written informed consent and were compensated monetarily for their participation. The research was performed in accordance with the Declaration of Helsinki.
Dementia diagnosis procedure
Dementia diagnosis within the whole Betula sample was set in 2015–2017 by a geropsychiatrist based on the DSM-IV criteria32, using previous medical history and results from neuroradiological examination. Additional information was obtained from health examination and neuropsychological test assessments as follows: Mini-Mental State Examination (MMSE) score below 23 or a drop by 3 points compared to results from previous test occasion, composite and memory test score ≥ 1.8 standard deviations below age-based norms with a decline in cognitive performance from the previous test occasion, self-reported memory impairment or observed signs of neurocognitive dysfunction at test occasions by nurses and psychologist conducting the testing. Evaluation of medical records was done at baseline and follow-up to increase the diagnostic precision and the reliability of the assessments33,34.
Episodic memory task
A face-name paired-association task including three task conditions; encoding, retrieval, and control, was performed during fMRI acquisition at both baseline and follow-up35,36. The face-name stimuli of the encoding condition consisted of an unknown face shown on a black background together with a fictional first name, forming a face-name pair. The names were popular first names, and the faces were digital color photographs with equal numbers of male and female faces. During encoding, participants were asked to remember the face-name pairs and press a button to indicate the item was seen (and to have similar motor responses across the three task conditions). During retrieval, the same faces were presented along with three letters, from which participants were asked to indicate by button press the letter that corresponds to the first letter of the previously encoded name. The left letter corresponded to the index finger, the middle letter to the middle finger, and the right letter to the ring finger. Participants were instructed to guess when not remembering a name. During the active control, participants were asked to indicate with a button press, as quick as possible, when a circle presented at the center of the screen changed to a cross35,36. The task was divided into six encoding blocks, six retrieval blocks, and eight blocks of the control task, each 20 s long. For encoding and retrieval, four stimuli were presented per block with a duration of four seconds, separated by the presentation of a cross. The interstimulus intervals was randomized between 1.5 and 4.5 s to allowed for both event-related and blocked analyses. The total fMRI scan time was 10 min for each participant.
fMRI acquisition
At both baseline and follow-up, the fMRI data were collected on a 3 T General Electric (GE) Discovery MR750 scanner with a 32-channel head coil. The functional images were acquired with a gradient-echo EPI sequence according to the following parameters: TR = 2.0 s, TE = 30 ms (ms), flip angle = 80°, 37 slices (3.9 mm thick), 96 × 96 matrix, FOV = 25 × 25 cm. To allow for saturation of the fMRI signal, ten dummy scans were collected and discarded prior to experimental image acquisition. Cushions inside the head coil were used to minimize subject head movement. E-Prime (Psychology Software Tools) was used for stimulus presentation and recording of responses from a MR-compatible response pad. In addition, structural T1-weighted images were acquired with a 3D fast spoiled gradient echo sequence (180 slices with a 1 mm thickness, TR: 8.2 ms, TE: 3.2 ms, flip angle: 12°, FOV: 25 × 25 cm).
fMRI analyses
The fMRI data were preprocessed and analyzed using SPM12 (Statistical Parametric Mapping, Wellcome Centre for Human Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm), implemented in MATLAB R2014b (MathWorks). SPM was run through an in-house program (DataZ). Preprocessing of the fMRI data included slice-timing correction, head movement correction by unwarping and realignment to the first image of each volume. The realigned images were then spatially normalized by co-registering participants’ functional images to their structural T1-weighted images. This was done separately for data from baseline and follow-up by segmenting each participants’ structural T1-weighted image into gray matter, white matter and cerebrospinal fluid components. The DARTEL37 toolbox was used to create a template image for each participant of the baseline and follow-up data, and also a group-level DARTEL template. The flow-field files that mapped the transformation from native space to DARTEL template and an affine transformation from DARTEL to Montreal Neurologic Institute (MNI) space was used for normalizing the fMRI data to MNI standard space with a 2 mm resolution, and smoothed with an 8 mm full width at half maximum Gaussian kernel. The data were high-pass filtered (128 s), and voxel-wise general linear models were set up for each participant using SPM12. In these models, block onsets and durations for each condition from the scanner task were included as regressor, modeled as a boxcar and convolved with a canonical hemodynamic response function (HRF). To remove movement-related artifacts, six realignment parameters from the motion correction preprocessing steps were included as covariates of no interest. Subject-level contrast images were generated, comparing the experimental conditions of the scanner task (encoding vs. control, and retrieval vs. control), separately for baseline and follow-up data. To identify hippocampal regions more activated during encoding and retrieval relative to the control task, the contrast images were carried on to random-effects group analyses using one sample t-tests of all subjects at baseline. Activation peaks were labeled as either anterior or posterior hippocampus31 depending on their location in MNI space relative to y = − 21 mm as suggested by Poppenk et al. for long-axis segmentation of the hippocampus in human neuroimaging38, demonstrated in Fig. 1. The coordinates of peak activations were retrieved from fMRI analyses to examine age-related changes in anterior and posterior hippocampus activity at encoding and retrieval, performed in our previous study31. Beta values from the hippocampus peak activations, identified from the whole-brain analysis of the baseline sample performed in our previous study31, were modeled separately for the left and right hippocampi. Left and right hippocampal volumes were measured using automatic subcortical segmentation (aseg) in FreeSurfer (v. 5.3), and adjusted to head size by dividing the hippocampal volume with the total intracranial volume (ICV).
Genotyping and construction of polygenic risk scores
The DNA extraction for single-nucleotide polymorphism (SNP) genotyping was done at the Institute of Human Genetics, University of Bonn, Germany. All DNA samples were genotyped using two types of Illumina arrays: Illumina Omni Express and Omni 1S Bead chip kits. Imputation of the raw genotypes was done using the ENIGMA2 protocol of the ENIGMA Consortium (http://enigma.ini.usc.edu/) to the 1000 genomes reference panel38 using minimac tools (version 2013.7.17)39. Post-imputation quality control (QC) was performed based on genotype call rate < 10%, minor allele frequency (MAF) < 1%, SNP missingness < 5%, and imputation info < 0.8. Before calculating the PRS, SNPs with ambiguous strand alignment were removed, as were SNPs within the APOE region (44.4–46.5 Mb on chromosome 19 on the hg19 assembly). Thereafter, PRS for AD were calculated using the summary statistics from a AD GWAS including 21,982 AD cases and 41,944 cognitively normal controls5. Linkage disequilibrium (LD) clumping was performed by discarding SNPs within 250 kb of, and in r2 ≥ 0.1 with another more significant SNP. The European sample of the 1000 Genomes Project phase 340 was used as LD reference panel for clumping, after removal of SNPs with genotype call rate < 1% and MAF < 1%. PRS were calculated for each individual by summing the alleles of the clumped SNPs weighted by the beta value from the GWAS5. PRS were constructed at p value thresholds of p < 5e−8, p < 0.05, and p ≤ 1, including 18, 41,305, and 290,660 SNPs, respectively.
Statistical analyses
To examine if hippocampal activation peaks were associated with scanner task performance or hippocampal volume at baseline, we performed linear regression analyses using the lm function in R. These regression analyses included hippocampal activity as covariate of interest, as well as sex, age, and sample as covariates of no interest. To examine the association of AD PRS and APOE ɛ4 with level and change in brain activation in anterior and posterior hippocampus, we performed linear mixed-effect models that were fitted in R using the lmer function available through the lme4 and lmerTest packages, using the extracted beta values as dependent variables. These models included the AD PRS as well as APOE ɛ4 carriage status (coded as 0/1 for non-carriers and carriers due to the low frequency of ɛ4 homozygotes) as covariates of interest, and sex, baseline age, adjusted hippocampal volume, sample, education, scanner task performace (number of hits) and the first five genetic principal components (PCs) for genetic ancestry as covariates of no interest. Age at each scanning session was used to estimate the slope, representing individualized aging-related change in activation over five years. Slope of all covariates was included in the models as well as random subject-specific intercepts. To test for association between AD genetics and differences on level and slope of performance on the scanner task (number of hits and response time), a linear mixed-effect model was used, including sex and age at baseline as covariates and APOE ɛ4 carriership or PRS as the independent variable and either hits or response time as the dependent variable. As an additional analysis, both behavioral and fMRI analyses of PRS were stratified into APOE ɛ4 carriers and non-carriers. All analyses were performed in R version 4.0.3. All continuous variables were z-transformed using the scale function in R, i.e., scaled to zero-mean and standard deviation (SD) of one.
Genetic prediction of AD risk
To evaluate whether APOE ɛ4 and/or PRS predict the risk of AD, we employed a Cause-specific hazard regression accounting for other dementia types and death as competing risks events. These analyses included 1,542 individuals, of which 791 remained healthy, 145 were diagnosed with AD, 121 were diagnosed with other dementia types, and 485 individuals died non-demented. In this type of competing risk regression analysis, the Cause-specific hazard function denotes the instantaneous rate of occurrence of the event (i.e., AD), in participants who are curently event-free. In a sensitivity Cause-specific hazard regression analysis, dementia types other than AD and vascular dementia were excluded due to the low number of cases, such as dementia not-otherwise specified (n = 13), dementia due to Parkinson's disease (n = 8), Lewy body dementia (n = 6), frontotemporal dementia (n = 2), and progressive supranuclear paralysis (n = 1). The sensitivity analysis also excluded individuals with low risk to develop dementia during the studied period (participants younger than 45 years at baseline, n = 184). Thus, sensitivity analysis (n = 1328) included 617 healthy individuals, 145 AD cases, 91 vascular dementia cases and 475 individuals who died non-demented. Cause-specific hazard regression analysis were performed with PRS excluding the APOE loci, based on p-thresholds of p < 5e−8, p < 0.05 and p ≤ 1. Time from baseline (in years) was used as the time scale. Regressions were adjusted for the first five PCs, APOE ε4 carriage, sex, age, and age-squared, and a sensitivity analysis included years of education. Dementia status, carriers of the APOE ε4 allele, and sex were included in the models as binary indicator variables (coded as 0/1).
Results
Genetic predictors of AD risk
We tested the effect of APOE ε4 carriership and PRS on risk for AD. The PRS was either calculated from all SNPs (PRSp≤1), SNPs that previously predicted AD at genome-wide significance (PRSp<5e−8), or SNPs that previously predicted AD at uncorrected significance level (PRSp<0.05). Both APOE ε4 and higher PRSp<5e−8 were significantly associated with increased risk of AD (Supplementary Table 1, and Supplementary Fig. 1). APOE ε4 carriers had a 3.8 times higher risk for AD compared to non-carriers (hazard ratio [HR] = 3.84, CI 2.7–5.4, p = 6.8e−15), while an increase in PRSp<5e−8 by one standard deviation from the mean was associated with a 1.2 times increase in the risk for AD (HR = 1.29, CI 1.080–1.549, p = 0.005). PRSp<5e−8 was similarly associated with risk for AD after controlling for years of education (HR = 1.29, CI 1.08–1.547, p = 0.006). In a sensitivity analysis excluding rare dementia types and individuals with low risk for developing AD (younger than 45 years old), the PRS also remained predictive of AD (HR = 1.28, CI 1.073–1.531, p = 0.006). PRSp<0.05 was marginally significantly associated with AD risk, while no effect was seen for PRSp≤1 (Supplementary Table 1).
Effects of genetic risk for AD on scanner task performance
Descriptives of the study population undergoing fMRI acquisition are presented in Table 1, sub-grouped according to APOE ε4 status. At baseline, there was no relation between APOE ε4 or AD PRS at any p value threshould with scanner task performance. Longitudinal analyses of scanner task performance revealed that AD PRSp<1 was significantly associated with more negative slopes in hits across the two fMRI sessions (t = − 3.37, p = 0.0008), whereas no effect was seen for APOE ε4 (t = − 1.37, p = 0.17). The AD PRS had no significant effect at more conservative PRS p value thresholds (PRSp<0.05, t = − 1.65, p = 0.0997, PRSp<5e−8, t = − 0.969, p = 0.333). Post-hoc analyses stratified by APOE status revealed that the effect of AD PRSp<1 on slope in task performance was strongest in APOE ε4 carriers (n = 78, t = − 2.8, p = 0.0056) , with a trend-level effect in non-carriers (n = 213, t = − 1.8, p = 0.067). A weak effect of PRS p<5e-8 was seen on response time (t = − 2.4, p = 0.02, i.e. shorter response time with higher risk), but no other genetic effects were observed for change in response time (all p > 0.1).
Effect of genetic risk for AD on age-related change in hippocampal activation
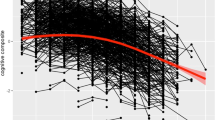
Prior to studies of AD genetics and hippocampal activation, we investigated if hippocampal activation peaks were associated with scanner task performance or hippocampal volume. We found that activation of the right and left anterior as well as the left posterior hippocampi were positively associated with number of hits (Supplementary Table 2), but not with hippocampal volume (Supplementary Table 3). During memory encoding, APOE ɛ4 carriers showed a more pronounced aging-related decrease in hippocampal activation relative to non-carriers. The strongest effect was observed in the right and left posterior hippocampi (Table 2, Fig. 2), while weaker effects in the same direction were observed in the anterior hippocampi (Table 2). During memory retrieval, a trend-level effect of APOE ɛ4 was seen in the right posterior hippocampus only (Table 2). No effects were observed for any PRS p value thresholds on level or slope in hippocampal activation (all p > 0.1). An example of the full model is presented in Supplementary Table 4 where the results for the left posteror hippocampus during encoding are shown. Post-hoc stratification based on APOE ɛ4 allele carriership or removing scanner task performance (hits and reaction time) from the model did not reveal any significant results of PRS on hippocampal activation (all p > 0.1).
Discussion
We have studied if the APOE ɛ4 allele and/or AD PRS influences aging-related changes in hippocampal functioning across four years, using longitudinal fMRI from a large population-based study2. Our hypothesis was that high genetic risk for AD would magnify our previously observed encoding-related decreases in anterior hippocampal activation with aging31. We found that APOE ɛ4 carriers had decreased hippocampal activation with increasing age, whereas no difference over time was seen in non-carriers (Fig. 2) or as a function of AD PRS. The effect of APOE ɛ4 was seen both during encoding and retrieval. The finding did not reflect a magnification of a general effect of age in the anterior hippocampi but was instead expressed most prominently in the posterior hippocampi. O’donogue et al.41 reviewed the current literature and described two plausible hypotheses where the role of APOE ɛ4 during aging either can be explained as pre-clinical AD pathology (‘prodromal hypothesis’) or direct genetic effects independent of subsequent development of AD (‘phenotypic hypothesis’). In favor of the phenotypic hypothesis, participants in the current study remained non-demented for at least a minimum of two years after the second fMRI assessment, and APOE ɛ4 carriers did not differ from non-carriers in task performance or hippocampal volume. Furthermore, previous fMRI studies have reported a stronger activation of the hippocampi in preclinical AD relative to controls42,43, while we instead observed less activation in APOE ε4 carriers relative to non-carriers. In addition, the AD PRS excluding the APOE locus did not affect hippocampal activity.
A plausible mechanism for an effect of APOE ε4 on age-related decreases in hippocampal functioning that is not secondary to prodromal AD, would be the role for APOE ε4 on breakdown of the blood–brain barrier (BBB)44. The link between APOE and BBB breakdown was recently shown to be specific to the hippocampal region and linked to cognitive decline through neurovascular uncoupling independently of amyloid-β and tau pathologies4. Similarly, our observation of a decrease in the BOLD signal in APOE ε4 carriers may represent an inability to increase blood flow sufficiently upon increased energy demand, rather than a decreased oxygen demand followed by reduced neuronal activity45,46. However, without longer follow-up data or AD-specific biomarkers, we cannot rule out the possibility that our results instead reflect prodromal AD processes, or a mixture. It should also be noted that APOE has a role in several pathways of importance for both AD and age-related cognitive decline, recently described in terms of “the APOE cascade hypothesis”47. In this model, the APOE ε4 gene variant gives rise to biochemical alterations of the APOE protein, resulting in a cascade of phenotypic effects including neuroinflammation, vascular dysfunction and neuropathology, underlying the clinical outcome48.
Although reduced hippocampal activation in healthy elderly has previously been linked to reduced memory performance31,49,50, lower activation in APOE ε4 was not linked to lower performance on the scanner task in this study. One explanation for this could be that we used a memory task at scanning that was optimized to elicit a strong hippocampal response, but not to reveal behavioral effects. In a previous publication on the same study population, we reported an APOE ε4 effect on age-related decreases in performance on more sensitive off-line cognitive tests8.
By segmentation of the hippocampi along the anterior–posterior axis, we could further observe that the effect of APOE ε4 was most prominent in the posterior hippocampi. However, since APOE ε4 also showed a slight effect on the activity in the anterior hippocampi, regional differences should be interpreted cautiously. APOE is expressed mainly in astrocytes and glia cells, but also in neurons of all hippocampal subunits (CA1-4 and DG)51. Potential differences of the effect of APOE ε4 on the BBB in hippocampal subunits have not yet been explored.
Based on previous studies showing effects of AD PRS on cognitive decline8 and level of hippocampal functioning in healthy aging29,30, we hypothesized that AD PRS would also predict decline in hippocampal activation. However, we did not observe such an effect for any of our three selected PRS p value thresholds. As our PRS sucessfully predicted both AD risk and scanner task performance, the lack of effect on brain activation is unlikely due to low power. Notably, only the PRSp≤1, i.e. including all SNPs, predicted decline in task performance across age, in line with our previous work on cognitive task performance8. This effect may result from the higher polygenicity of cognitive ability in general than of clinical AD52,53. The mechanisms behind variants with weaker association to AD could be mediated through a general effect on cognitive ability that impacts AD risk through educational attainment or other life-style factors. Thus, the genetic link to AD for PRSp≤1 is seemingly too distant to predict the disease in small independent studies, while a link to cognitive decline is more proximal. In contrast, the PRSp<5e−8 consisting of 18 SNPs excluding the APOE locus, captures genes with roles in AD-related processes, e.g. amyloid-β-, tau-, lipid transportations-, immune system-, and endocytosis pathways5,6. Our results suggest that those genes in general can predict AD risk in a small independent sample, but do not influence cognition or hippocampal functioning in healthy aging. However, one or a few of those genetic pathways might still play a role in normal neurocognitive aging individually.
Limitations
Given that pathological AD processes can start ten years before clinical detection of AD1, lack of longer diagnostic follow-up cannot rule out the possibility that some individuals who were assessed as cognitive healthy in fact were in a preclinical dementia stage. However, our two-year clinical follow-up was more extensive than most previous studies on aging in non-demented individuals. The number of individuals that both underwent fMRI and have developed AD in this sample is too small to restrict analyses to this specific sub-group. It should be noted that AD was defined clinically, and that clinically healthy individuals can manifest with AD-related biomarkers in the cerebrospinal fluid that meet the criterias for a biologically-defined AD54. We have examined a limited set of hippocampal regions. Although we argue that our areas of choice represent relevant parts of the hippocampi affected by aging, it cannot be ruled out that the analyses of additional regions would result in different outcomes.
In conclusion, our findings suggest an effect of APOE ɛ4, but not polygenic risk for AD, on longitudinal change in hippocampal functioning in healthy aging.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to lack of ethical permit for sharing sensitive person data, but are available from the corresponding author on reasonable request.
References
Scheltens, P. et al. Alzheimer’s disease. Lancet 397, 1577–1590 (2021).
Nyberg, L. et al. Biological and environmental predictors of heterogeneity in neurocognitive ageing: Evidence from Betula and other longitudinal studies. Ageing Res. Rev. https://doi.org/10.1016/j.arr.2020.101184 (2020).
Serrano-Pozo, A., Das, S. & Hyman, B. T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 20, 68–80 (2021).
Montagne, A. et al. APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature 581, 71–76 (2020).
Kunkle, B. W. et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 51, 414–430 (2019).
Jansen, I. E. et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 51, 404–413 (2019).
Bellou, E., Stevenson-Hoare, J. & Escott-Price, V. Polygenic risk and pleiotropy in neurodegenerative diseases. Neurobiol. Dis. 142, 104953 (2020).
Kauppi, K., Rönnlund, M., Nordin Adolfsson, A., Pudas, S. & Adolfsson, R. Effects of polygenic risk for Alzheimer’s disease on rate of cognitive decline in normal aging. Transl. Psychiatry https://doi.org/10.1038/s41398-020-00934-y (2020).
Li, J.-Q., Tan, L., Wang, H.-F., Tan, M.-S., Tan, L., Xu, W. et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer’s disease: A systematic review and meta-analysis of cohort studies. J. Neurol. Neurosurg. Psychiatry 1–9 (2016).
Murray, A. N., Chandler, H. L. & Lancaster, T. M. Multimodal hippocampal and amygdala subfield volumetry in polygenic risk for Alzheimer’s disease. Neurobiol. Aging 98, 33–41 (2021).
Foley, S. F. et al. Multimodal brain imaging reveals structural differences in Alzheimer’s disease polygenic risk carriers: A study in healthy young adults. Biol. Psychiatry 81, 154–161 (2017).
Foo, H. et al. Associations between Alzheimer’s disease polygenic risk scores and hippocampal subfield volumes in 17,161 UK Biobank participants. Neurobiol. Aging 98, 108–115 (2021).
Gorbach, T. et al. Longitudinal association between hippocampus atrophy and episodic-memory decline in non-demented APOE ε4 carriers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 12, 1–9 (2020).
Machulda, M. M. et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 61, 500–506 (2003).
Johnson, S. C. et al. Activation of brain regions vulnerable to Alzheimer’s disease: The effect of mild cognitive impairment. Neurobiol. Aging 27, 1604–1612 (2006).
Petrella, J. R. et al. Mild cognitive impairment: Evaluation with 4-T functional MR imaging. Radiology 240, 177–186 (2006).
Dickerson, B. C. et al. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 56, 27–35 (2004).
Dickerson, B. C. et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411 (2005).
Celone, K. A. et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. J. Neurosci. 26, 10222–10231 (2006).
Hämäläinen, A. et al. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging 28, 1889–1903 (2007).
Kircher, T. T. et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J. Neurol. Neurosurg. Psychiatry 78, 812–818 (2007).
Yassa, M. A. et al. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage 51, 1242–1252 (2010).
Filippini, N. et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. U. S. A. 106, 7209–7214 (2009).
Trachtenberg, A. J., Filippini, N. & Mackay, C. E. The effects of APOE-ε4 on the BOLD response. Neurobiol. Aging 33, 323–334 (2012).
Bookheimer, S. Y. et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 343, 450–456 (2000).
Trivedi, M. A. et al. fMRI activation during episodic encoding and metacognitive appraisal across the lifespan: Risk factors for Alzheimer’s disease. Neuropsychologia 46, 1667–1678 (2008).
Dennis, N. A. et al. Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimer’s Dement. 6, 303–311 (2010).
Adamson, M. M., Hutchinson, J. B., Shelton, A. L., Wagner, A. D. & Taylor, J. L. Reduced hippocampal activity during encoding in cognitively normal adults carrying the APOE e{open}4 allele. Neuropsychologia 49, 2448–2455 (2011).
Chandler, H. L., Hodgetts, C. J., Caseras, X., Murphy, K. & Lancaster, T. M. Polygenic risk for Alzheimer’s disease shapes hippocampal scene-selectivity. Neuropsychopharmacology 45, 1171–1178 (2020).
Xiao, E. et al. Late-onset Alzheimer’s disease polygenic risk profile score predicts hippocampal function. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2, 673–679 (2017).
Nyberg, L., Andersson, M., Lundquist, A., Salami, A. & Wåhlin, A. Frontal contribution to hippocampal hyperactivity during memory encoding in aging. Front. Mol. Neurosci. 12, 1–11 (2019).
American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed., Text Revision), Text Revision (2000).
Rönnlund, M., Sundström, A., Adolfsson, R. & Nilsson, L. G. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimer’s Dement. 11, 1385–1392 (2015).
Nilsson, L.-G. et al. Betula: A prospective cohort study on memory, health and aging. Aging Neuropsychol. Cogn. 11, 134–148 (2004).
Persson, J., Kalpouzos, G., Nilsson, L., Ryberg, M. & Nyberg, L. Preserved hippocampus activation in normal aging as revealed by fMRI. Hippocampus 21, 753–766 (2011).
Pudas, S. et al. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J. Neurosci. 15, 8668–8677 (2013).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007).
Poppenk, J., Evensmoen, H. R., Moscovitch, M. & Nadel, L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 17, 230–240 (2013).
The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2016).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G. R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2013).
O’Donoghue, M. C., Murphy, S. E., Zamboni, G., Nobre, A. C. & Mackay, C. E. APOE genotype and cognition in healthy individuals at risk of alzheimer’s disease: A review. Cortex 104, 103–123 (2018).
Talwar, P., Kushwaha, S., Chaturvedi, M., Mahajan, V. Systematic review of different neuroimaging correlates in mild cognitive impairment and Alzheimer’s disease. Clin. Neuroradiol. 953–967 (2021).
Li, H. J. et al. Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: A meta-analysis of 75 fMRI studies. Hum. Brain Mapp. 36, 1217–1232 (2015).
Halliday, M. R. et al. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 36, 216–227 (2016).
Wåhlin, A. & Nyberg, L. At the heart of cognitive functioning in aging. Trends Cogn. Sci. 23, 717–720 (2019).
Iadecola, C. The neurovascular unit coming of age: A journey through neurovascular coupling in health and disease. Neuron 96(17–42), 48 (2017).
Martens, Y. A. et al. ApoE cascade hypothesis in the pathogenesis of Alzheimer’s disease and related dementias. Neuron 110, 1304–1317 (2022).
Nyberg, L. Functional brain imaging of episodic memory decline in ageing. J. Intern. Med. 281, 65–74 (2017).
Tromp, D., Dufour, A., Lithfous, S., Pebayle, T. & Després, O. Episodic memory in normal aging and Alzheimer disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 24, 232–262 (2015).
Salami, A., Eriksson, J. & Nyberg, L. Opposing effects of aging on large-scale brain systems for memory encoding and cognitive control. J. Neurosci. 32, 10749–10757 (2012).
Pu-Ting, X. et al. Specific regional transcription of apolipoprotein E in human brain neurons. Am. J. Pathol. 154, 601–611 (1999).
Zhang, Q., Sidorenko, J., Couvy-duchesne, B., Marioni, R. E., Wright, M. J., Goate, A. M., et al. Risk prediction of late-onset Alzheimer’s disease implies an oligogenic architecture. Nat. Commun. 1–11 (2020).
Hill, W. D., Marioni, R. E., Maghzian, O., Ritchie, S. J., Hagenaars, S. P., McIntosh, A. M., et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol. Psychiatry 1–13 (2018).
Jack, C. R. et al. Prevalence of biologically vs clinically defined alzheimer spectrum entities using the national institute on aging-Alzheimer’s association research framework. JAMA Neurol. 55905, 1–10 (2019).
Acknowledgements
We would like to thank all study participants for their important contribution, and Micael Andersson for technical support during the fMRI analyses.
Funding
Open access funding provided by Umea University. This work was supported by a grant to KK from the Kempe foundation (reference no SMK-1865) as well as from the Swedish Research Council (Grant No 2017–03011). The Betula data collection was supported by a scholar-grant from Knut and Alice Wallenberg’s (KAW) foundation to LN.
Author information
Authors and Affiliations
Contributions
S.H., E.K., and F.S.H. performed analyses and wrote parts of the manuscript. L.N. designed the fMRI paradigm, and advised on fMRI data analysis and interpretation of results. K.K. was responsible for idea, concept, and design of the study, performed analyses and drafted the manuscript. All authors contributed with reviewing and editing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Håglin, S., Koch, E., Schäfer Hackenhaar, F. et al. APOE ɛ4, but not polygenic Alzheimer’s disease risk, is related to longitudinal decrease in hippocampal brain activity in non-demented individuals. Sci Rep 13, 8433 (2023). https://doi.org/10.1038/s41598-023-35316-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35316-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.