Abstract
Transgenic corn, Zea mays (L.), expressing insecticidal toxins such as Cry1Fa, from Bacillus thuringiensis (Bt corn) targeting Ostrinia nubilalis (Hübner) (Lepidoptera: Crambidae) resulted in over 20 years of management success. The first case of practical field-evolved resistance by O. nubilalis to a Bt corn toxin, Cry1Fa, was discovered in Nova Scotia, Canada, in 2018. Laboratory-derived Cry1Fa-resistance by O. nubilalis was linked to a genome region encoding the ATP Binding Cassette subfamily C2 (ABCC2) gene; however, the involvement of ABCC2 and specific mutations in the gene leading to resistance remain unknown. Using a classical candidate gene approach, we report on O. nubilalis ABCC2 gene mutations linked to laboratory-derived and field-evolved Cry1Fa-resistance. Using these mutations, a DNA-based genotyping assay was developed to test for the presence of the Cry1Fa-resistance alleles in O. nubilalis strains collected in Canada. Screening data provide strong evidence that field-evolved Cry1Fa-resistance in O. nubilalis maps to the ABCC2 gene and demonstrates the utility of this assay for detecting the Cry1Fa resistance allele in O. nubilalis. This study is the first to describe mutations linked to Bt resistance in O. nubilalis and provides a DNA-based detection method that can be used for monitoring.
Similar content being viewed by others
Introduction
Genes encoding insecticidal toxins from the bacterium Bacillus thuringiensis (Bt) Berliner were engineered into crops to safely and sustainably manage insect pests1. Since their first year of commercialization in 1996, the global area planted to Bt crops increased from 1.1 million to 109 million hectares in 20191. Intoxication with Bt toxins follows a series of steps that involve activation of the toxin by midgut proteases, binding of the activated toxin to receptors on the brush membrane of the midgut, and formation of pores that result in feeding cessation followed by death2,3,4. Changes to these steps can result in Bt resistance, which is a genetically based decrease in susceptibility of an insect population to a Bt toxin4,5.
Cases of field-evolved resistance to Bt crops increased from three in 2005 to 43 in 20205. The cases of field-evolved Bt resistance have involved 15 pest species and ten Bt toxins in nine countries5; yet, the mechanisms of resistance and causal genetic mutations are known only for Spodoptera frugiperda (J. E. Smith) with field-evolved Cry1F resistance and Pectinophora gossypiella (Saunders) with field-evolved Cry1Ac and Cry2Ab resistance6,7,8,9,10. The first case of practical field-evolved resistance to Bt corn, Zea mays L., by O. nubilalis was discovered in 2018 in Nova Scotia, Canada, involving Cry1Fa11. Two additional Cry1Fa-resistant populations have since been detected in Quebec and Manitoba12. Early warning signs of resistance to Cry1Ab, Cry1A.105, and Cry2Ab2 toxins have also been detected in eastern Canada12. These findings suggest that Cry1Fa resistance may be spreading, and additional O. nubilalis resistance issues may be on the rise. Understanding the mechanisms involved in field-evolved Bt resistance may help in the development of much needed DNA-based monitoring and detection methods.
Understanding of the genetics of Bt resistance has primarily come from studies on laboratory-derived Bt resistance which may differ from field-evolved resistance. The most commonly described method of Bt resistance is a change in toxin binding to midgut receptors4. Mutations in ATP binding cassette (ABC) genes have been linked to resistance against Cry1, Cry2, and Cry3 toxins6,7,13,14,15,16,17,18,19,20,21. In the case of Cry1Fa, resistance has been linked to the ABC subfamily C member 2 (ABCC2) gene in many lepidopteran species such as Bombyx mori (L.), Spodoptera frugiperda (J. E. Smith), O. furnacalis (Guenée), and O. nubilalis6,7,13,14,15,16,17,18. Furthermore, Vellichirammal et al. found genes likely involved in Bt toxin mode of action such as ABCC2, aminopeptidase, and several other serine proteases were down regulated in Cry1Fa susceptible but not in laboratory-derived Cry1Fa-resistant O. nubilalis when exposed to Cry1Fa toxin18; however, these genes were not investigated further. The ABCC2 gene produces a transmembrane protein that typically consists of two transmembrane domains, connected by extracellular and intracellular loops (ECL and ICL, respectively), and two nucleotide binding domains (NBD). Mutations to ABCC2 causing Cry toxin resistance include nucleotide insertions, substitutions, and deletions, resulting in changes to ECL 2 and 4, NBD2, and protein truncation6,7,13,14,15,22,23,24.
Genetic linkage mapping indicated that laboratory-derived Cry1Fa-resistance in O. nubilalis was controlled by a single quantitative trait locus and involved a single gene17,25. In this study, we combined previous research on ABCC2 genes with a classic candidate gene approach to investigate field-evolved Cry1Fa-resistance in O. nubilalis. The specific aims of this study were to (1) determine the heritability and level of recessivity of the field-evolved Cry1Fa-resistance trait in O. nubilalis, (2) identify mutations in the candidate gene linked to field-evolved Cry1Fa-resistance, and (3) develop a DNA-based Cry1Fa resistance monitoring method.
Our results indicate that the field-evolved Cry1Fa-resistance trait in O. nubilalis is highly recessive. Sequencing analysis revealed multiple SNPS in the ABCC2 gene which strongly support ABCC2 as a causal agent of Cry1Fa resistance. Furthermore, Cry1Fa-resistance in O. nubilalis is genetically linked to a single amino acid change in one of the extracellular loops and a protein truncating mutation in a known Cry toxin receptor. This study is the first to describe mutations linked to Bt resistance in O. nubilalis and provides a DNA-based detection method that can be used for monitoring. Additionally, this work demonstrates the value and efficacy of candidate gene approaches for identifying mechanisms of resistance, such as Bt resistance in economically important agricultural systems.
Results
Toxin-overlay diet bioassays
The susceptibility of four O. nubilalis strains to Cry1Fa was determined using a toxin-overlay diet bioassay with a non-treated control (0 ng Cry1Fa cm−2) and a diagnostic concentration of 200 ng Cry1Fa cm−2. Mortality of ON-S1 and ON-S2 was 100% with the diagnostic concentration, which was significantly greater than the mortality of NS-R and QC-R (< 25%) and the mortality of all strains in the non-treated control (< 15%) (Table 1). There was no difference in mortality between concentrations for NS-R and QC-R (Table 1). Based on these results, ON-S1 and ON-S2 were deemed susceptible to Cry1Fa, while NS-R and QC-R were deemed resistant.
Introgression experiment
An introgression experiment was conducted to establish a back-cross strain that is an isoline of ON-S1 but resistant to Cry1Fa. To do this, ON-S1 was crossed with NS-R1 and the heterozygote F1 where then interbred. Following this, F2, selected for their resistance to Cry1Fa, were back-crossed with ON-S1. This process was repeated 5 times, as shown in Table 2 and described in materials and methods section. The diagnostic concentration of Cry1Fa caused 100% mortality among the offspring of ON-S1 crossings (ON-S1 × Fn), but mortality among the interbred crossing (Fn × Fn) was significantly lower, ranging between 70–76% (Table 2). Mortality in all back-crossed strains was significantly greater when exposed to the Cry1Fa diagnostic concentration relative to the non-treated control (Table 2). These results demonstrate that the Cry1Fa resistance allele is recessive and inheritance followed Mendelian expectations for a single gene (Table 2). BC-R was established by interbreeding F10 individuals that survived exposure to the Cry1Fa diagnostic concentration. Exposing this strain to the diagnostic concentration of Cry1Fa caused 17% mortality which did not differ from the non-treated control (Table 2).
Sequencing
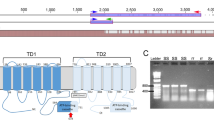
The ABCC2 gene of O. nubilalis contained 24 predicted exons, totaling 4,089 bp of coding sequence. We sequenced ~ 4.0 Kb (> 98%) of the ABCC2 coding region from ON-S1 and NS-R. In total, we found 20 SNPs (~ 1 per 200 bp) between resistant and susceptible individuals and relative to the reference gene. Most SNPs were synonymous substitutions that did not alter the amino acid sequence, and a few were fixed between susceptible and resistant strains. Presented in Table 3 are six nonsynonymous SNPs, one of which is a two nucleotide base deletion (SNP5), fixed between resistant and susceptible strains and the predicted amino acid change. The predicted location of these SNPs on the ABCC2 protein are presented in Fig. 1. SNP4 resulted in an amino acid change from glycine in the susceptible strains to serine in the resistant strains. This change is predicted to be on ECL4 (Fig. 1). The two nucleotide base deletion (SNP5) induced a frameshift and a premature stop codon at amino acid 982, truncating the protein and resulting in the loss of the 11th and 12th transmembrane helices and NBD2 (Fig. 1).
Representative diagram of the predicted Cry1Fa-susceptible ABCC2 protein structure and location of the SNPs in the Cry1Fa-resistant ABCC2 protein in Ostrinia nubilalis. Diagram is not drawn to scale, including the length of the extra-cellular loops (ECL) and the localization of the nucleotide binding domain (NBD). The diagram includes the approximate location of the SNPs in Cry1Fa-resistant ABCC2 protein (red arrows). Numbers indicate the amino acid residue at that location. The dotted lines and light grey colour for the Transmembrane Domain 2 represent the predicted region missing in the truncated Cry1Fa-resistant ABCC2 protein.
The sequenced data were compared to the publicly available sequence of the laboratory-derived Cry1Fa-resistant O. nubilalis described in Vellichirammal et al.18 where we found 18 of the 20 identified SNPs present between the susceptible and Cry1Fa-resistant strains. Of the nonsynonymous SNPS, all five were fixed between the susceptible and laboratory-derived Cry1Fa-resistant strains (Table 3). However, SNP5 documented in our field-evolved Cry1Fa-resistant strains was not present in the laboratory-derived Cry1Fa-resistant O. nubilalis described in Vellichirammal et al.18 (Table 3). A non-synonymous SNP downstream of SNP6 was present in the laboratory-derived Cry1Fa-resistant strain described in Vellichirammal et al.18 (hereinafter referred to as SNPV) that resulted in protein truncation due to a premature stop codon, with the predicted loss of the NBD2 (Table 3).
Genotyping assays
A restriction enzyme digest genotyping assay was developed to detect the six non-synonymous SNPs found in the sequenced resistant strains. The Cry1Fa-resistant strains were homozygous for the six non-synonymous SNPs, while the non-synonymous SNPs were only detected at a frequency of 0.04 in ON-S1 and 0.00 for ON-S2 (Table 4). BC-R was also homozygous for the six non-synonymous SNPs (Table 4).
Randomly selected individuals from the last back-cross assay were genotyped and digest results are presented in Fig. 2. Restriction enzyme digests of randomly selected individuals from the last back-cross assay showed that individuals who survived exposure to non-treated control were in Hardy–Weinberg equilibrium (χ2 (2, n = 25) = 0.4400, P = 0.8025), with 24% homozygous for the six non-synonymous SNPs associated with Cry1Fa susceptibility (p2), 52% heterozygous (2pq), and 24% homozygous for the six non-synonymous SNPs (q2) (Fig. 2a). The percentage of genotyped individuals that died after exposure to diagnostic concentration were 56, 40, and 4% for homozygous susceptible (p2), heterozygous (2pq), and homozygous resistant (q2), respectively (Fig. 2b), which deviated from Hardy–Weinberg equilibrium (χ2 (2, n = 25) = 6.1200, P = 0.0469). Individuals that survived exposure to diagnostic concentration were 100% homozygous for the six non-synonymous SNPs (q2) (Fig. 2c).
Allele frequencies of six non-synonymous SNPs associated with field-evolved Cry1Fa-resistance in Ostrinia nubilalis for the (a) survived exposure to non-treated control, (b) died after exposure to diagnostic concentration (200 ng Cry1Fa cm−2), and (c) survived exposure to diagnostic concentration (200 ng Cry1Fa cm−2) of the F2BC-RxON-S1 as determined using restriction enzyme digests. Black, grey, and white bars indicate homozygotes for the alleles associated with Cry1Fa susceptibility (p2), heterozygotes (2pq), and homozygotes for the alleles associated with Cry1Fa resistance (q2), respectively.
Discussion
Field-evolved Cry1Fa-resistance in O. nubilalis is closely linked to mutations in the ABCC2 gene which functions as a receptor for the Cry1Fa toxin in susceptible insects. This is the first study to describe mutations linked to Bt resistance in O. nubilalis. Analogous comparisons between laboratory-derived and field-evolved Cry1F resistant O. nubilalis show striking similarities at the gene-level and with some of the detected mutations. Genotyping results using a Cry1Fa-resistant back-crossed strain provide support for linkage of six mutations to field-evolved Cry1Fa-resistance in O. nubilalis. The linkage tests detected no heterozygote survival among the back-crossed larvae exposed to the Cry1Fa toxin, suggesting that the resistance trait is completely recessive. Survival of approximately 25% of back-crossed individuals exposed to a diagnostic dose of Cry1Fa indicates that the resistance trait is controlled by a single gene. This result is similar to that reported within a laboratory-derived Cry1Fa-resistant O. nubilalis strain17.
A single nucleotide substitution that resulted in an amino acid change in ECL4 was present among the field-derived resistant strains in this study and in the laboratory-derived Cry1Fa-resistant strain18. ECL4 has been suggested as a potential binding site for Cry1 toxins as studies have shown that mutations in ECL4 confer resistance to Cry1 toxins in S. frugiperda, S. exigua, Helicoverpa armigera (Hübner), and B. mori13,14,15,16,24,26,27. However, the mutation described in this study requires further investigation to better indicate its link to the binding site of Cry1Fa on the ABCC2 protein.
Another mutation described in this study is a two nucleotide base deletion that induces a frameshift, resulting in a premature stop codon, truncating the protein, and resulting in the loss of the last third of the second transmembrane domain including the NBD2. These nucleotide deletions were also present among the BC-R and QC-R strains tested, but not in the laboratory-derived Cry1Fa-resistant strain. However, SNPV was present in the laboratory-derived Cry1Fa-resistant strain and also resulted in protein truncation, with the predicted loss of the NBD2. High-level resistance to Cry1Fa was documented using a CRISPR-mediated ABCC2 gene knockout that resulted in the loss of both ECL4 and NBD2 in O. furnacalis and S. exigua23,28. Furthermore, mutational changes associated with field-evolved Cry1Fa-resistant S. frugiperda from Puerto Rico and Florida resulted in ABCC2 protein truncation with the loss of the second transmembrane domain, including ECL4 and NBD26,7. Mutations in NBD2, including the NBD2-deletion, did not, on their own, alter Cry1Fa susceptibility in baculovirus-free insect cell expression of ABCC2 variants of S. frugiperda13. Using functional genetic testing such as baculovirus-free insect cells expressing the mutations documented in this study, either alone or in combination, can help determine the mutations responsible for Cry1Fa-resistance in O. nubilalis and determine the mechanisms behind Cry1Fa resistance.
ABCC2 has been described as a binding receptor for Cry1Ab, Cry1Ac, and Cry1A.105, in addition to Cry1Fa15,16,29. Mutations to ABCC2 resulting in Cry1Fa-resistance may also alter O. nubilalis susceptibility to other Cry1 toxins if resistance was due to an alteration of the shared binding sites30,31. CRISPR-mediated ABCC2 gene knockout in O. furnacalis resulted in over 300-fold resistance to Cry1Fa and 4–eightfold resistance to Cry1Ab and Cry1Ac23. Knocking out ABCC2 in S. frugiperda conferred over 120-fold resistance to Cry1Fa and Cry1Ab32. In a cell expression study of ABCC2 mutations documented in S. frugiperda, single nucleotide substitutions in ECL4 conferred over 630-fold resistance to Cry1Fa and over ninefold resistance to Cry1Ab and Cry1A.10513. ABCC2 as a shared receptor among Cry1 toxins was also documented in other Lepidopteran species such as B. mori, H. armigera, and S. exigua15,16,22,24,28.
The frequency of the Cry1Fa resistance allele before the adoption of Cry1Fa corn in NS is unknown as Bt resistance monitoring was not conducted in the Maritimes region before 2018. The Cry1Fa resistance allele frequency (0.04) observed in the ON-S1 strain in the present study is greater than that reported in the U.S. corn belt (> 10–3)33. The Cry1Fa resistance allele frequency in U.S. corn belt was estimated using an F1, where field-collected individuals were crossed with a resistant laboratory strain, and F2 screening approach to estimate Cry1Fa resistance allele frequency. The results revealed the presence of the Cry1Fa resistance allele frequency at higher than estimated levels even during the first year of commercialization of Cry1Fa corn; however, there was no indication of that frequency increasing over the 7 years of the study33. The origin of this allele is also unclear. However, B. thuringiensis is ubiquitous in the environment34 and contact with this bacterium throughout O. nubilalis evolution may have resulted in the presence of alleles conferring resistance to Bt toxins3. Evolution of resistance is likely to capitalize on alleles already present in the population3.
The analogous comparison of the genetic basis of Bt resistance between laboratory-derived and field-evolved Cry1Fa resistance in O. nubilalis show a great deal of similarities at both the gene-level and with some of the detected mutations. To our knowledge, only one other study conducted analogous comparisons between laboratory-derived and field-evolved Bt resistance, finding striking similarities between the two strains of P. gossypiella9. These similarities highlight the usefulness of laboratory selection for identifying genes important in field-evolved Bt resistance, but may not necessarily capture the specific mutations in those genes. The present study is the first to describe mutations in O. nubilalis linked to Bt resistance and is one of only three studies to identify molecular genetic basis of practical Bt resistance. Using the genotyping method described in this study, changes in the frequency of Cry1Fa-resistance across the range of O. nubilalis can be detected, allowing for a more rapid implementation of Bt resistance management strategies.
Methods and material
Insect strains
Strains of O. nubilalis used in this study were initiated from fifth-instar larvae collected from commercial corn fields. Cry1Fa-susceptible strains were collected from Winger, ON (ON-S2) and Delaware, ON (ON-S1) and in 2010 and 2016, respectively. Cry1Fa-resistant strains were collected from Masstown, NS (NS-R) and Saint-Mathieu-de-Boloeil, QC (QC-R) in 201912 (SI Appendix, Table S1). Larvae were reared in 36 cm diameter plastic tubs containing meridic diet with corrugated cardboard rings placed above the diet surface as described by Smith et al.11. Once the majority of larvae had pupated in the rings, they were transferred to cages (30 cm × 30 cm × 60 cm) with waxed paper sheets placed on top of the mesh roof for oviposition. Waxed sheets were replaced daily and placed into plastic sweater boxes lined with moistened paper towels. All rearing stages and bioassays were maintained at 26:18 °C, 60% RH, and 16:8 (L:D) h photoperiod.
Toxin-overlay diet bioassays
The susceptibility of O. nubilalis strains to Cry1Fa was determined using toxin-overlay diet bioassays. Diet was prepared in the same manner as for rearing11. Using a repeater pipette, 1 mL of diet was dispensed into each well of a 128-well bioassay tray (Bio-16, CD International, Pitman, NJ) resulting in a surface area of 2.0 cm2. After the diet has solidified, wells were covered with adhesive ventilated lids (Frontier Agricultural Sciences, Newark, DE), and stored at 4 °C. Lyophilized toxin standard containing > 95% purity of activated Cry1Fa, produced from Escherichia coli (Migula) containing the Cry1F gene, was obtained from M. Pusztai-Carey (Case-Western University, Cleveland, OH), and stored at −80 °C. Cry1Fa toxin was constituted using 10 mM CAPS (3-cyclohexylamino-1-propane sulfonic acid) buffer solution with the pH adjusted to 10.5 using 10 N NaOH. Cry1Fa toxin solution was diluted using 10 mM CAPS buffer solution to a diagnostic concentration of 200 ng cm−2. The non-treated control was treated with 10 mM CAPS buffer solution only. The diagnostic concentration of Cry1Fa or the non-treated control solution was applied to the diet surface of each well in 30 μl aliquots using a repeater pipette. Trays were tilted in all directions to cover the entire diet surface with solution. Trays were kept in a fume hood as the solvent component of the solution evaporated. A single neonate (< 24 h old) larva was transferred with a fine-paint paint brush to each well of the bioassay tray. Infested wells were covered with adhesive lids, placed into rearing conditions, and covered with cardboard to prevent condensation. A total of 24 larvae were treated per solution per bioassay. Individual larval mortality and weight were recorded after seven days. Stunted larvae that were alive but weighed less than 0.1 mg were considered dead. Each bioassay was repeated five times for each strain.
Introgression experiment
Adults from ON-S1 and NS-R were crossed following the cage method described earlier in the following configurations: 50 ON-S1♀ × 50 NS-R♂ and 50 NS-R♀ × 50 ON-S1♂. Progeny (F1) of the crosses were allowed to mate and their offspring (F2) were exposed to a diagnostic concentration of Cry1Fa as described for the toxin-overlay diet bioassays. Survivors of Cry1Fa exposure were then back-crossed to the ON-S1 strain. This process was repeated five times resulting in a Cry1Fa-resistant strain (BC-R) that is > 96% genetically similar to the ON-S1 strain. Toxin-overlay diet bioassays were conducted with the BC-R strain as described above with 24 and 72 larvae exposed to the zero and diagnostic concentration of Cry1Fa, respectively, and replicated five times. Restriction enzyme genotype analysis was conducted on 25 randomly selected larvae from all five replications from each of the following categories: survived exposure to 0 ng Cry1Fa cm−2, died after exposure to 200 ng Cry1Fa cm−2, and survived exposure to 200 ng Cry1Fa cm−2.
Sequencing
To determine single nucleotide polymorphisms (SNPs) in the ABCC2 gene associated with susceptibility and resistance to the Cry1Fa toxin, RNA samples from two ON-S1 and two NS-R individuals were sequenced. RNA was extracted using TRIzol™ Plus RNA purification kit (Invitrogen™, Waltham, MA) following manufacturer’s instructions. Samples were amplified with SuperScript™ IV One-Step RT-PCR System kit (Invitrogen™, Waltham, MA) on a VeritiPro™ Thermal Cycler (Applied Biosystems™, Waltham, MA) and primers were designed as described below to amplify the target locus. The cycling conditions included reverse transcription at 50 °C for 10 m, followed by initial denaturation at 98 °C for 2 m, then 40 cycles at 98 °C for 10 s, 55 °C for 10 s, and 72 °C for 1 m, and a final extension at 72 °C for 5 min. Since the sequence for the O. nubilalis ABCC2 gene is unknown, we used the sequence from a closely related species, O. furnacalis (NCBI Accession #MN783372) to search the O. nubilalis draft genome (NCBI Assembly ASM892168v1). Search results via BLAST provided a single genomic location for ABCC2 on scaffold SWFO01000764.1 at positions 115,342–132,146 (16,804 bp). PCR primers were developed to amplify the coding region (cDNA) at this locus in 5 separate but overlapping amplicons ranging from 774–918 bp in length (SI Appendix, Table S2). Coding used to obtain the ECB ABCC2 gene is available at https://github.com/mikesovic/Farhan_etal_ABCC2. PCR products were loaded into 1.5% agarose gels and separated at 100 V for 1 h. Resulting fragments sizes were estimated by comparison to a DirectLoad™ 50-bp ladder (Sigma-Aldrich, St. Louis, MO). Amplicons were sent to the Advanced Analysis Centre (University of Guelph, Guelph, ON) for Sanger sequencing. Sequence data were aligned using BioEdit 7.2, to the O. nubilalis ABCC2 gene and SNPs consistent between ON-S1 and NS-R individuals were documented. Sequencing data were compared to the publicly available sequences of the laboratory-derived Cry1Fa-resistant O. nubilalis described in Vellichirammal et al.18.
Open reading frame (ORF) and amino acid sequence of the ABCC2 protein were predicted using the ORF finder software (https://www.ncbi.nlm.nih.gov/orffinder/) and ExPASy Bioinformatics resource portal (http://web.expasy.org/translate/), respectively. The transmembrane topology was predicted using the Phobius web server35. BLAST search of the NCBI Conserved Domains Database (http://www.ncbi.nlm.nih.gov/cdd) was used to predict the ATP binding cassettes.
Genotyping assay
Based on the sequencing data, a restriction enzyme digest method was developed to determine the presence and linkage of SNPs in resistant individuals. Genomic DNA was extracted from fifth instars of the ON-S1, ON-S2, NS-R, and QC-R strains, as well as from first instars of the ON-S1 × NS-R and BC-R strains using QuickExtract™ DNA Extraction Solution (Lucigen Corporation, Middleton, WI) following the manufacturer’s protocol and quantified using Ultraspec 2100 pro spectrometer (Biochrom Ltd, Cambridge, UK). Samples were amplified using GoTaq G2 Green Master Mix (Promega, Madison, WI), with forward and reverse primers (Sigma-Aldrich, St. Louis, MO) (SI Appendix, Table S3) using VeritiPro™ Thermal Cycler (Applied Biosystems™, Waltham, MA). The cycling conditions included initial denaturation at 95 °C for 5 m, then 37 cycles at 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 40 s, and a final extension at 72 °C for 5 min. The amplicons were digested using the restriction enzymes listed in SI Appendix, Table S3 (New England Biolab, Whitby, ON) for 1.5 h following the manufacturer’s protocol. Five of these restriction enzymes cut the resistant allele at the indicated position, while enzyme MspI cut the susceptible allele at SNP1 (SI Appendix, Table S3). Digest products were loaded on 1.5% agarose gel and separated using 100 V for 1 h. The resulting fragment sizes were analysed using a DirectLoad™ 50-bp ladder (Sigma-Aldrich, St. Louis, MO). Protocol describing the PCR and digest methods is available in SI Appendix.
Data analyses
Toxin-overlay diet bioassays
Mortality at the diagnostic concentration of Cry1Fa was analysed using PROC GLIMMIX in SAS v. 9.4 (SAS Institute, Cary, NC) with strain as the fixed effect and replicate as a random effect. Mortality data followed a beta distribution with a logit link and values of 0 and 1 were replaced with 0.0001 and 0.9999, respectively36. Tukey’s Honestly Significant Difference (HSD) test was used for multiple treatment comparisons and the α level for statistical significance was set at 0.05.
Genotyping assay
A chi-squared test was conducted using PROC FREQ in SAS v. 9.4 (SAS Institute, Cary, NC) to determine if the genotypes for the individuals tested in the back-cross experiment were in Hardy–Weinberg equilibrium.
Data availability
The data generated and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Tabashnik, B. E. & Carriere, Y. Global patterns of resistance to Bt crops highlighting Pink Bollworm in the United States, China, and India. J. Econ. Entomol. 112, 2513–2523. https://doi.org/10.1093/jee/toz173 (2019).
Heckel, D. G. The essential and enigmatic role of ABC transporters in Bt resistance of noctuids and other insect pests of agriculture. Insects 12, 1. https://doi.org/10.3390/insects12050389 (2021).
Heckel, D. G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect. Biochem. Physiol. 104, 21673. https://doi.org/10.1002/arch.21673 (2020).
Jurat-Fuentes, J. L., Heckel, D. G. & Ferre, J. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 66, 121–140. https://doi.org/10.1146/annurev-ento-052620-073348 (2021).
Tabashnik, B. E., Fabrick, J. A. & Carriere, Y. Global patterns of insect resistance to transgenic Bt crops: The first 25 years. J. Econ. Entomol. https://doi.org/10.1093/jee/toac183 (2023).
Banerjee, R. et al. Large genomic deletion linked to field-evolved resistance to Cry1F corn in fall armyworm (Spodoptera frugiperda) from Florida. Sci. Rep. 12, 13580. https://doi.org/10.1038/s41598-022-17603-3 (2022).
Banerjee, R. et al. Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci. Rep. 7, 10877. https://doi.org/10.1038/s41598-017-09866-y (2017).
Jakka, S. R. K. et al. Field-evolved mode 1 resistance of the fall armyworm to transgenic Cry1Fa-expressing corn associated with reduced Cry1Fa toxin binding and midgut alkaline phosphatase expression. Appl. Environ. Microbiol. 82, 1023–1034. https://doi.org/10.1128/AEM.02871-15 (2016).
Fabrick, J. A. et al. Alternative splicing and highly variable cadherin transcripts associated with field-evolved resistance of pink bollworm to bt cotton in India. PLoS One 9, e97900. https://doi.org/10.1371/journal.pone.0097900 (2014).
Fabrick, J. A., Li, X., Carriere, Y. & Tabashnik, B. E. Molecular genetic basis of lab- and field-selected Bt resistance in pink bollworm. Insects 14, 1. https://doi.org/10.3390/insects14020201 (2023).
Smith, J. L., Farhan, Y. & Schaafsma, A. W. Practical resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) to Cry1F Bacillus thuringiensis maize discovered in Nova Scotia, Canada. Sci Rep 9, 18247. https://doi.org/10.1038/s41598-019-54263-2 (2019).
Smith, J. L. & Farhan, Y. Monitoring resistance of Ostrinia nubilalis (Lepidoptera: Crambidae) in Canada to Cry toxins produced by Bt corn. J. Econ. Entomol. https://doi.org/10.1093/jee/toad046 (2023).
Franz, L., Raming, K. & Nauen, R. Recombinant expression of ABCC2 variants confirms the importance of mutations in extracellular loop 4 for Cry1F resistance in fall armyworm. Toxins (Basel) 14, 1. https://doi.org/10.3390/toxins14020157 (2022).
Liu, Y. et al. SfABCC2 transporter extracellular loops 2 and 4 are responsible for the Cry1Fa insecticidal specificity against Spodoptera frugiperda. Insect. Biochem. Mol. Biol. 135, 103608. https://doi.org/10.1016/j.ibmb.2021.103608 (2021).
Pinos, D., Martinez-Solis, M., Herrero, S., Ferre, J. & Hernandez-Martinez, P. The Spodoptera exigua ABCC2 Acts as a Cry1A receptor independently of its nucleotide binding domain II. Toxins (Basel) 11, 1. https://doi.org/10.3390/toxins11030172 (2019).
Tanaka, S. et al. Bombyx mori ABC transporter C2 structures responsible for the receptor function of Bacillus thuringiensis Cry1Aa toxin. Insect. Biochem. Mol. Biol. 91, 44–54. https://doi.org/10.1016/j.ibmb.2017.11.002 (2017).
Coates, B. S. & Siegfried, B. D. Linkage of an ABCC transporter to a single QTL that controls Ostrinia nubilalis larval resistance to the Bacillus thuringiensis Cry1Fa toxin. Insect. Biochem. Mol. Biol. 63, 86–96. https://doi.org/10.1016/j.ibmb.2015.06.003 (2015).
Vellichirammal, N. N. et al. Transcriptional analysis of susceptible and resistant European corn borer strains and their response to Cry1F protoxin. BMC Genom. 16, 558. https://doi.org/10.1186/s12864-015-1751-6 (2015).
Tay, W. T. et al. Insect resistance to Bacillus thuringiensis Toxin Cry2Ab Is conferred by mutations in an ABC transporter subfamily a protein. PLoS Genet. 11, e1005534. https://doi.org/10.1371/journal.pgen.1005534 (2015).
Pauchet, Y., Bretschneider, A., Augustin, S. & Heckel, D. G. A P-glycoprotein is linked to resistance to the Bacillus thuringiensis Cry3Aa toxin in a leaf beetle. Toxins (Basel) 8, 1. https://doi.org/10.3390/toxins8120362 (2016).
Gahan, L. J., Pauchet, Y., Vogel, H. & Heckel, D. G. An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet 6, e1001248. https://doi.org/10.1371/journal.pgen.1001248 (2010).
Flagel, L. et al. Mutational disruption of the ABCC2 gene in fall armyworm, Spodoptera frugiperda, confers resistance to the Cry1Fa and Cry1A.105 insecticidal proteins. Sci. Rep. 8, 7255. https://doi.org/10.1038/s41598-018-25491-9 (2018).
Wang, X. et al. CRISPR-Mediated Knockout of the ABCC2 Gene in Ostrinia furnacalis Confers High-Level Resistance to the Bacillus thuringiensis Cry1Fa Toxin. Toxins (Basel) 12, 1. https://doi.org/10.3390/toxins12040246 (2020).
Xiao, Y. et al. Mis-splicing of the ABCC2 gene linked with Bt toxin resistance in Helicoverpa armigera. Sci. Rep. 4, 6184. https://doi.org/10.1038/srep06184 (2014).
Coates, B. S. et al. A single major QTL controls expression of larval Cry1F resistance trait in Ostrinia nubilalis (Lepidoptera: Crambidae) and is independent of midgut receptor genes. Genetica 139, 961–972. https://doi.org/10.1007/s10709-011-9590-0 (2011).
Atsumi, S. et al. Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori. Proc. Natl. Acad. Sci. USA 109, E1591-1598. https://doi.org/10.1073/pnas.1120698109 (2012).
Sato, R., Adegawa, S., Li, X., Tanaka, S. & Endo, H. Function and role of ATP-binding cassette transporters as receptors for 3D-Cry toxins. Toxins (Basel) 11, 1. https://doi.org/10.3390/toxins11020124 (2019).
Huang, J. et al. Evaluation of five candidate receptors for three Bt toxins in the beet armyworm using CRISPR-mediated gene knockouts. Insect. Biochem. Mol. Biol. 121, 103361. https://doi.org/10.1016/j.ibmb.2020.103361 (2020).
Crespo, J. G., Goller, F. & Vickers, N. J. Pheromone mediated modulation of pre-flight warm-up behavior in male moths. J. Exp. Biol. 215, 2203–2209. https://doi.org/10.1242/jeb.067215 (2012).
Hernandez-Rodriguez, C. S., Hernandez-Martinez, P., Van Rie, J., Escriche, B. & Ferre, J. Shared midgut binding sites for Cry1A105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS ONE 8, e68164. https://doi.org/10.1371/journal.pone.0068164 (2013).
Hernandez-Martinez, P. et al. Comparison of in vitro and in vivo binding site competition of Bacillus thuringiensis Cry1 proteins in two important maize pests. Pest Manag. Sci. 78, 1457–1466. https://doi.org/10.1002/ps.6763 (2022).
Jin, M. et al. Two ABC transporters are differentially involved in the toxicity of two Bacillus thuringiensis Cry1 toxins to the invasive crop-pest Spodoptera frugiperda (J. E. Smith). Pest Manag. Sci. 77, 1492–1501. https://doi.org/10.1002/ps.6170 (2021).
Siegfried, B. D. et al. Estimating the frequency of Cry1F resistance in field populations of the European corn borer (Lepidoptera: Crambidae). Pest Manag. Sci. 70, 725–733. https://doi.org/10.1002/ps.3662 (2014).
Martin, P. W. & Travers, R. Worldwide Abundance and Distribution of Bacillus thuringiensis Isolates. Appl Environ. Microbiol. 55, 2437–2442 (1989).
Kall, L., Krogh, A. & Sonnhammer, E. L. Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucl. Acids Res. 35, W429-432. https://doi.org/10.1093/nar/gkm256 (2007).
Bowley, S. R. A hitchhiker's guide to statistics in biology. Generalized linear mixed model edition., (Plants et al., Inc., 2015).
Acknowledgements
The authors wish to thank the growers for allowing us to collect O. nubilalis from their farms. We are thankful to our collaborators Dr. Julien Saguez, Centre de Recherche sur les Grains (CÉROM) and Sonny Murray and Caitlin Congdon, Perennia Food and Agriculture, Inc for collecting O. nublialis in their respective provinces. We are grateful to Marianne Pushtai-Carey for supplying Cry1Fa toxin. We thank Emily Glasgow for her work on developing the back-cross population. This research project was funded by Bayer CropScience Inc., the National Sciences and Engineering Research Council of Canada Alliance grant, Pioneer Hi-Bred International Inc., Syngenta Canada Inc., the Manitoba Crop Alliance, Grain Farmers of Ontario, The Atlantic Grains Council, Perennia Food and Agriculture Inc., Centre de Recherche sur les Grains (CÉROM), and the Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA), through the Ontario Agri-Food Innovation Alliance.
Author information
Authors and Affiliations
Contributions
Y.F. and A.P.M designed the work; Y.F., M.G.S., and A.P.M. contributed to data collection; Y. F. and A.M.P contributed to data analysis and interpretation; Y.F. drafted the manuscript; All authors contributed to the critical revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Farhan, Y., Smith, J.L., Sovic, M.G. et al. Genetic mutations linked to field‐evolved Cry1Fa-resistance in the European corn borer, Ostrinia nubilalis. Sci Rep 13, 8081 (2023). https://doi.org/10.1038/s41598-023-35252-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-35252-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.