Abstract
A perennial criticism of provisioning ecotourism is that it alters the natural behavior and ecology of the target species by providing an artificial food source. Here we evaluate its impact on the long-term site fidelity patterns of tiger sharks in French Polynesia. We hypothesized that a significant impact of provisioning would lead to (1) increases in individual site fidelity over time, and (2) an increase in the number of resident individuals over time. Of 53 individuals photo-identified and monitored during > 500 dives over five years, 10 individuals accounted for > 75% of all sightings, whereas 35 sharks were sighted very infrequently. Even the most frequently observed tiger sharks exhibited overall low fidelity at the site and showed no increase in site fidelity over time. Furthermore, the number of tiger sharks sighted during each dive did not increase. The observed patterns of tiger shark sightings were best explained by natural movements, including general roaming within home ranges along the coastline and seasonal migrations. Despite the apparent lack of impact of provisioning ecotourism on tiger shark ecology in Tahitian waters, it would be prudent to implement a strict code of conduct during any future provisioning activities to maximize the safety of participants and animals involved.
Similar content being viewed by others
Introduction
The tiger shark Galeocerdo cuvier (Péron & Lesueur 1822) is a large (up to 550 cm Total Length)1 apex predator in tropical and temperate waters2. Although routinely present in coastal shelf, reef and slope habitats2, tiger sharks are typically rarely encountered by scuba divers in the wild, excepted under baited conditions2,3. This species is currently near threatened2 with population declines evident in several locations including Northeast Australia4 and the Arabian Seas Region5. Their large size and reputation as a potentially dangerous apex predator make tiger sharks a popular target species for shark ecotourism in locations such as Fiji, South Africa and the Bahamas, where in the latter it has contributed to a shark watching industry that has generated $800 million over a 20 years period6,7,8. The economic viability of shark tourism depends on predictable and sustainable sightings of the target species9 which in the case of tiger sharks typically requires the use of attractants ranging from simple olfactory stimulus (chumming) to active feeding of individuals present in the area10.
Shark feeding for ecotourism purposes is a controversial activity11,12. A primary concern is that provisioning may teach sharks to associate humans with food and thereby increase the risk of shark bites13,14. However, to date no demonstrable increase in shark bites has been observed at or around provisioning sites many of which have shark feeding regulations that strictly limit the amount of food that can be consumed15,16,17. A further criticism is that artificial provisioning (i.e. humans deliberately feeding wildlife) alters the natural ecology and behavior of the target species by increasing site fidelity (i.e. time spent at provisioning sites) at the expense of wider-ranging movements18,19. This effect is inherently difficult to assess because shark site fidelity naturally varies both inter- and intra-specifically15,20,21. For example, significant intraspecific variability in site fidelity is naturally present in silky sharks Carcharhinus falciformis (Bibron 1839)22, sicklefin lemon sharks Negaprion acutidens (Rüppel 1837)23, and bull sharks Carcharhinus leucas (Valenciennes in Müller & Henle 1839)24 with some individuals showing high fidelity at specific sites while others are only occasional transient visitors. The limited evidence available to date suggests that tiger shark movement patterns are not significantly affected by provisioning25. Despite these inherent challenges, it is important for us to understand how provisioning activities impact shark behavior and ecology in order to effectively manage shark ecotourism activities to ensure adequate protection of these iconic species10,26.
The major concerns surrounding provisioning ecotourism are particularly pertinent to tiger sharks because they are one of the three main shark species routinely implicated in unprovoked bites on humans (second only to white sharks Carcharodon carcharias (Linnaeus 1758))27 and show considerable natural variability in movement patterns, often showing fidelity to a specific “home” island, but also able to roam thousands of kilometers into open-ocean28. Tiger sharks variously show high site-fidelity to core use areas (CUA) and wide-ranging movements in coastal habitats, and wide-ranging movements and multi-month residence times in open-ocean in both the Indo-Pacific and Atlantic regions29,30,31,32. Seasonal migrations by tiger sharks have been documented in the Atlantic, Indian and Pacific Oceans31,33,34, and appear to be linked to sea surface temperature (SST), especially in more temperate regions toward the latitudinal limits of tiger shark distribution31,34. Tiger sharks occur in SSTs ranging from 18 to 33 °C but coastal abundance and swimming performance of tiger sharks are both highest at ~ 22 °C, suggesting thermal constraints on performance may regulate this species’ distribution35,36,37.
Tiger sharks were a focal species at the “Vallée Blanche” (VB) shark ecotourism dive site in Tahiti (French Polynesia) where shark provisioning activities occurred from 2011 until October 2017 when shark feeding was officially banned (Law of the Country 2017–25). The regular ecotourism activities at the VB site from October 7, 2012 to October 9, 2017 provided an opportunity to study the long-term impacts of provisioning on tiger shark abundance and site fidelity. We hypothesized that an impact of provisioning on tiger shark movement patterns would result in (i) increased fidelity of individual tiger sharks over time (i.e. visiting on more days and being present for longer periods), and (ii) an increase in the number of individuals observed per dive over time.
Results
Tiger shark relative abundance
A total of 1027 tiger shark sightings were recorded at the VB site during 544 dives conducted between 7 October 2012 and 9 October 2017. Among the 1027 sightings, 855 (84%) were of individuals previously documented by Bègue et al.38. The total number of individuals observed simultaneously during each dive ranged from 0 to 8 (mean = 1.87 ± 1.30 SD). The relative abundance of tiger sharks per dive had a non-normal, non-homoscedastic distribution (Shapiro–Wilk normality test & Bartlett test of homogeneity of variances, p-values < 0.05). The number of bait drums present per dive ranged from 0 to 4 (mean = 1.38 ± 0.80 SD). Bait drum numbers had a non-normal (Shapiro–Wilk normality test, p-value < 0.0001) but homoscedastic (Bartlett test of homogeneity of variances, p-value = 0.86) distribution. No significant correlation was found between the relative abundance of tiger sharks observed per dive and number of bait drums simultaneously present (Spearman correlation, S = 9.48 × 105, p-value = 0.17, rho = 0.10; Supplementary Fig. S1). The relative abundance of tiger sharks observed per dive did not change significantly across the years sampled (Spearman correlation, S = 2.71 × 107, p-value = 0.66, rho = 0.019, Supplementary Fig. S2). However, within years the relative abundance of tiger sharks observed per dive was significantly higher during austral winter than austral summer (Kruskal–Wallis rank sum test, \(\chi\)2 = 24.95, df = 1, p-value < 0.0001, Supplementary Fig. S3).
Site fidelity & use
Tiger sharks (n = 53) observed at the VB site were predominantly (94%) female and primarily sexually mature (64% of females, 100% of males). The sex ratio did not display any significant variation between years (Spearman correlation, S = 2.28 × 107, p-value = 0.13, rho = − 0.068) or between seasons (Kruskal–Wallis rank sum test, \(\chi\)2 = 0.52, df = 1, p-value = 0.47). The total number of sightings of each individual ranged from 1 to 109 with a mean value of 16.11 ± 28.17 (Table 1). The maximum overall SFI (SFIg) value for any photo-identified individual was 0.2 (Fig. 1, Table 1), and the overall mean SFIg value was 0.03 ± 0.05. Five female tiger sharks with SFIg > 0.1 accounted for > 55% of the total sightings at VB despite collectively representing less than 10% of 53 photo-identified tiger sharks (Fig. 1, Table 1). The ten most frequently sighted (SFIg > 0.05) individuals were all females and collectively accounted for more than 76% of the total sightings (Fig. 1, Table 1). Thirty-five (66%) of 53 photo-identified tiger sharks were sighted 5 times or less and 18 (34%) of sharks were sighted only once (Fig. 1, Table 1).
Site Fidelity Indices calculated from sightings of uniquely identifiable G. cuvier at the Vallée Blanche ecotourism site between the 7 October 2012 and the 9 October 2017. Individuals are ordered by decreasing SFIg. Standard Deviation (SD) represents variation between years. Mature individuals are represented in red and non-mature in blue.
Individual annual site fidelity (SFIa) varied with no consistent patterns evident across years (Spearman correlation, S = 2.15 × 106, p-value = 0.76, rho = − 0.020, Supplementary Fig. S4) or between individuals (Kruskal–Wallis rank sum test, \(\chi\)2 = 134.14, df = 52, p-value = 3.48 × 10−9, no pairwise comparisons were significant with Dunn’s test, Supplementary Fig. S5). Some tiger sharks were sighted during every year of the 6-year survey whereas others were absent for up to a year between sightings (Fig. 1, Table 1). The maximum annual SFIa value was 0.413, obtained for TF01M during the first year of study, and the minimum annual SFI value was 0. The 10 most frequently sighted individuals (SFIg > 0.05) did not show any significant variation in SFIa between years (Spearman correlation, S = 1.61 × 104, p-value = 0.66, rho = 0.067, Supplementary Fig. S6).
Despite a general trend of more frequent tiger shark sightings during the austral summer, some intra-specific variation in seasonal patterns of tiger shark sightings was also evident (Fig. 3). For example, sightings of 8 of the 10 most frequently sighted tiger sharks (SFIg > 0.05, 28–109 occurrences), peaked during austral summer and were lowest during austral winter, whereas TF03M was present most frequently between January and September and TF22nM between December and June (Table 1, Fig. 3). All sharks except TF04M showed a strong intra-individual variability in presence pattern depending on the month of the year. Furthermore, 7 sharks were rarely present (presence probability < 0.05) or completely absent from the ecotourism site for several months. Other individuals (e.g. TF04M) showed less seasonal variation in presence, or had a high year round probability of presence (e.g. TF13nM) (Fig. 2). No significant difference in presence between the number of non-mature and mature individuals was observed between austral winter and austral summer (\(\chi\)2 test: \(\chi\)2 = 0.32, df = 1, p-value = 0.57).
Discussion
We found no evidence of either increasing site fidelity or established resident behavior (SFI > 0.5)39 by tiger sharks at the VB ecotourism site despite over 6 years of regular provisioning at this location. Overall site fidelity (SFIg) of each of the 53 uniquely identified tiger sharks was low (⩽ 0.2) and the maximum annual site fidelity (SFIa) for any individual was 0.413 and declined over time. Furthermore, no significant rise in the number of tiger sharks sighted simultaneously during each dive was apparent. This observed lack of significant impact of regular provisioning on tiger shark fidelity in French Polynesia is consistent with the results of previous studies on highly migratory sharks including tiger sharks in the Caribbean25, white sharks Carcharodon carcharias at cage diving sites in South Africa40 and Mexico41,42, and bull sharks Carcharhinus leucas in Fiji24, none of which found any demonstrable influence of provisioning on site fidelity. By contrast, previous studies of less mobile species such as sicklefin lemon sharks Negaprion acutidens16,23 and blacktip reef sharks Carcharhinus melanopterus (Quoy & Gaimard 1824)19 did detect increasing fidelity over time at other Polynesian provisioning sites. These interspecific differences in susceptibility to provisioning effects on fidelity may stem from fundamental differences in the spatial ecology of these various species. Species displaying naturally high levels of mobility and strong variability in movement patterns (e.g. tiger sharks) may simply be harder to retain at provisioning sites than highly reef-associated sharks (e.g. blacktip reef sharks).
Inter-individual differences in tiger shark SFIg observed at VB may also simply reflect natural variability in movement patterns and home range locations. Previous telemetry studies in Hawaii have shown tiger sharks occupy core-structured home ranges around oceanic islands15,28,30,43,44. Although their entire home range may span multiple oceanic islands and extend into open-ocean, individual tiger sharks spend most of their time within a smaller Core Use Area (CUA) often associated with one stretch of coastline, and multiple individuals routinely occupy highly overlapping CUAs within the same general area28. Similar tiger shark home range characteristics may exist around the oceanic Windward Islands of French Polynesia. Thus, the most frequently sighted tiger sharks in this current study may have CUAs that include VB but still exhibited overall low fidelity at the ecotourism site because they continually roam back and forth within a CUA that may be as large as the entire North coast of Tahiti, similar to the observations made in Hawaii’s oceanic islands28. Conversely, tiger sharks that were only occasionally sighted may occupy CUAs that do not include VB and their sporadic appearances at the ecotourism site reflect less-frequent wider-ranging movements outside their CUAs. Thus, the observed patterns of tiger shark sightings at provisioning ecotourism sites could be entirely explained by natural home range structure and there is no evidence that provisioning is significantly modifying their movement patterns or CUA locations. Satellite tagging could be used to empirically define tiger shark CUAs around Tahiti island to evaluate this hypothesis.
Although home range structure alone could reasonably explain observed SFI patterns it is important to consider a potential confounding effect of individual shark “boldness” on those patterns. We cannot rule out the possibility that bolder sharks willing to come closer to divers were seen most frequently whereas other more timid tiger sharks were also attracted to the site by provisioning activities but simply remained out of sight for most of the time. Previous studies with juvenile bull sharks showed some individuals being detected in riskier locations more often than others45 reinforcing the possibility that variable boldness among individuals could be contributing to the observed patterns of tiger shark use of the VB site. Boldness can be related to body size in sharks with larger animals generally dominant at feeding aggregations and smaller sharks excluded until larger individuals are satiated46,47. However, at the VB site three subadult tiger sharks were among the 10 most frequently sighted individuals and overlapped in presence with large adults indicating that body size alone does not explain observed SFI patterns. Additional telemetry studies of tiger shark movements around Tahiti could help to clarify whether individual boldness influences diver-based surveys of tiger shark presence. Furthermore, a recent study suggests that individual tiger sharks displaying high levels of testosterone or plasma steroids may be correlated with longer time spent at the provisioning site48. Thus, impact of physiological state on site occupancy must be further explored in future studies.
The significant and consistent seasonal fluctuations in tiger shark abundance at the VB ecotourism site are a further indication that their natural movement patterns are not significantly influenced by provisioning. The distinctive high-low winter-summer pattern of tiger shark abundance at VB is consistent with seasonal migrations of the kind previously documented in tiger sharks in other locations without artificial provisioning29,33, and also in tiger sharks and other species at other provisioning ecotourism sites15,22,23,24,49,50. The drivers of these seasonal movements could include reproduction or seasonal prey abundance. For example, the VB site is predominantly frequented by adult females, similar to Tiger Beach in Bahamas51, which may migrate seasonally for mating or pupping purposes. Seasonal variations in tiger shark abundance in other areas have been related to foraging on seasonal prey-aggregations such as fledging albatross Phoebastria spp. in Hawaii30 or loggerhead turtles Caretta caretta (Linnaeus 1758)52 in the North Atlantic ocean. Although the drivers of seasonal cycles of tiger shark abundance at the VB site are unknown, their continued manifestation despite consistent provisioning demonstrates that artificial feeding does not overwhelm these natural patterns.
Wildlife provisioning is controversial largely because it can be deleterious to animals and people53. Examples of wildlife provisioning leading to negative outcomes include the death of a 9-year old boy resulting in the culling of 31 Australian dingoes Canis lupus dingo (Linnaeus 1758) in the Fraser Island National Park54, a dangerous increase in the number of Indonesian Komodo dragons Varanus komodoensis (Ouwens 1912) in provisioning areas55, and provisioned Thai macaques Macaca fascicularis (Raffles 1821) showing more aggressive behaviours than non-provisioned individuals towards humans56. Thus, potential impacts of shark provisioning must be carefully considered in order to avoid negative consequences to sharks and people. Our current results support those of other recent studies suggesting a general lack of chronic or irreversible impacts of feeding activities on sharks24,25,40,50,57,58,59. In fact the positive experiences of participants in these activities leads to important economic benefits and increased support for shark conservation60,61,62. However, careful management and adherence a strict code of conduct are essential to keep shark provisioning ecotourism safe and ecologically sustainable10,13,16,58,63,64. For example, regulations restricting shark provisioning permits to certified operators, might be a way to penalize non-compliance with management plans. Such measures are intended to increase the safety of participants and target species and are already successfully applied in Australia with the endangered whale shark Rhincodon typus (Smith 1828) as well as with the critically endangered sand tiger shark Carcharias taurus (Rafinesque 1810)65,66,67. Our results suggest that provisioning ecotourism at the VB site did not significantly influence tiger shark site fidelity and we suggest that this practice can be kept safe and sustainable through appropriate regulations including prohibiting risky practices such as hand feeding16,63.
Methods
Study area
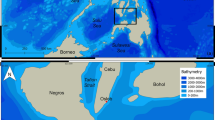
The study was carried out at the “Vallée Blanche” (VB) dive site (S 17.542°; W 149.624°), located on the outer slope of the barrier reef close to the Papeete pass on North-West coast of Tahiti (French Polynesia) (Fig. 3A,B). This site covers an area of approximately 40,000 m2 characterized by a central valley of sand and coral debris at a depth of between 15 and 20 m, surrounded by pinnacles of hard corals, and adjacent to a steep drop-off and exposed to strong and variable currents38. Diving centers initiated shark feeding activity in 2011 in order to concentrate sharks in a particular area and to increase the probabilities of sighting six different species: blacktip reef sharks Carcharhinus melanopterus, grey reef sharks Carcharhinus amblyrhynchos (Bleeker 1856), whitetip reef sharks Triaenodon obesus (Rüppel 1837), tawny nurse sharks Nebrius ferrugineus (Lesson 1831), sicklefin lemon sharks Negaprion acutidens and tiger sharks Galeocerdo cuvier (Fig. 3C). Overall, 53 tiger sharks (50 females and 3 males) were individually photo-identified using unique characteristics, including skin pattern such as stripes or deep scars, wounds, or damaged dorsal or pectoral fins38,47. Individual identities were carefully validated via a thorough cross comparison of different body areas.
Map of the study area with (A) Its location off Tahiti, French Polynesia; (B) Its satellite view (Google Earth); (C) An underwater view of shark provisioning (photo credit: Michel Bègue). White circle: location of the Papeete pass, when red circle represents the city of Papeete, capital of Tahiti island.
Data collection
Shark feeding activity occured year-round at VB, with almost daily provisioning sessions during Polynesian austral winter (May to October) but more sporadic provisioning during austral summer due to frequently unfavorable ocean conditions (high swell) and fewer tourists, but did occur whenever sea conditions were suitable. Sharks were attracted to the site using 4–6 yellowfin tuna heads Thunnus albacares (Bonnaterre 1788) contained in metal washing-machine drums (to prevent bait consumption) placed on the seabed at a depth of 18 m. The attraction process began each day at 8 AM on the first dive of the day. A second dive usually took place at 11 AM without food release, followed by a third dive at 2 PM, where the bait was released (head by head) at the end of the dive and fed to the waiting sharks. This schedule was based on the typical activity schedules of local dive centers. Dives were typically 45 min in duration. During the sampling period, VB was visited by 10 dive centers of which up to 4 attracted sharks at the same time with multiple bait drums distributed across the dive spot68. There was no coordination of these activities among dive operators but all dive centers involved in provisioning sharks visited VB almost daily for at least one dive. Due to the difficulty in evaluating the exact amount of tuna heads provided when several diving operators were sharing VB, the bait quantity was evaluated using the number of visible drums present. All dives were considered as independent in the following analysis.
Relative abundance, defined as the number of tiger sharks sighted per dive, identity, and size were recorded by an expert diver (M. Bègue) with more than 500 dives on the site. All the sharks identified in a previously developed database, were individually recognizable by based on their unique markings38. The size was assessed visually by the expert, and confirmed with the use of stereo-laser photogrammetry on several animals (n = 12), which showed an acceptable error < 5% of the estimated size. This non-invasive technique consists of two perfectly parallel laser dots calibrated at a specific distance apart, allowing the total length of the animal to be calculated from pictures69,70. ImageJ was used to compare the number of pixels between the two dots and the number of pixels for the total length of the shark. The sexual maturity status of each individual was estimated from their total lengths, with females > 3.30 m TL and males > 2.92 m TL considered to be sexually mature71. Each individual was assigned an alphanumeric identification code consisting of “T” for the species (Tiger), “F” (Female) or “M” (Male) for the sex, a two-digit number linked to the ranking of the first observation at VB (first tiger observed = 01, second observed = 02 etc.), and “M” (Mature) or “nM” (non-Mature) indicating the sexual maturity of the animals.
Evaluation of potential deleterious effects of provisioning
Data analyses were conducted in R (V 4.2.2)72 with the significance level set to 0.05. Shapiro–Wilk tests were used to check the normality and Bartlett tests to check the homoscedasticity of the distributions of different variables studied, resulting in non-parametric tests being selected for the data analyses. We used a Spearman correlation test to compare baiting level, quantified as the number of drums simultaneously present during the dive, with tiger shark relative abundance. We used Kruskal–Wallis rank sum tests to evaluate yearly and seasonal variations in tiger shark relative abundance with relative abundance as response variable and the year of sampling or the season as potential explanatory variables. Wilcoxon tests were used to make pairwise comparisons of significant results in order to identify periods of the year where tiger sharks were sighted significantly more frequently by divers at the VB site.
We calculated the global site fidelity index (SFIg) and the annual site fidelity index (SFIa) for each photo-identified individual by dividing the number of dives where the shark was seen by the total number of dives conducted during the respective sampling period. SFI values range from 0 to 1, with low values characterizing tiger sharks with a low site fidelity at VB and vice versa. An individual was considered as “resident” for a given year if the calculated SFIa was equal to or higher than 0.5, and as “inter-annual resident” when it showed a SFIa equal or higher than 0.5 over consecutive years40. To explore potential intra-specific differences in site use during the sampling period, we used Durbin Watson tests to examine the autocorrelation in sightings of individuals with > 25 total observations during the 5 years of sampling. For the 10 most-frequently sighted photo-identified tiger sharks, we generated response curves of monthly probability of presence deduced from the binomial presence/absence (1/0) data and fitted with a Loess smoother. \(\chi\)2 tests were used to evaluate whether presence patterns of the most frequently sighted sharks was significantly associated with either maturity status (mature and non-mature) or season (austral summer or austral winter). If significant, post hoc analysis based on residuals of Pearson’s \(\chi\)2 test for count data were used to further characterize the relationships. For the 10 most-frequently sighted tiger sharks, we used Kruskal–Wallis tests to evaluate the influence of study year on SFIa. Significant results were further assesses using Wilcoxon tests paiwise comparisons.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Meyer, C. G. et al. Growth and maximum size of tiger sharks (Galeocerdo cuvier) in Hawaii. PLoS ONE 9(1), e84799 (2014).
Ferreira, L. C. & Simpfendorfer, C. Galeocerdo cuvier. The IUCN Red List of Threatened Species 2019: e.T39378A2913541 (2019).
Kohler, N. E. & Turner, P. A. Shark tagging: A review of conventional methods and studies. Environ. Biol. Fishes 60, 191–224 (2001).
Holmes, B. J. et al. Declining trends in annual catch rates of the tiger shark (Galeocerdo cuvier) in Queensland, Australia. Fish. Res. 129, 38–45 (2012).
Jabado, R. W. & Spaet, J. L. Elasmobranch fisheries in the Arabian Seas Region: Characteristics, trade and management. Fish Fish. 18(6), 1096–1118 (2017).
Topelko, K. N. & Dearden, P. The shark watching industry and its potential contribution to shark conservation. J. Ecotour. 4(2), 108–128 (2005).
Gallagher, A. J. & Hammerschlag, N. Global shark currency: The distribution, frequency, and economic value of shark ecotourism. Curr. Issue Tour. 14(8), 797–812 (2011).
Brena, P. F., Mourier, J., Planes, S. & Clua, E. Shark and ray provisioning: Functional insights into behavioral, ecological and physiological responses across multiple scales. Mar. Ecol. Prog. Ser. 538, 273–283 (2015).
Whittaker, D. Capacity norms on bear viewing platforms. Hum. Dimens. Wildl. 2, 37–49 (1997).
Gallagher, A. J. et al. Biological effects, conservation potential, and research priorities of shark diving tourism. Biol. Cons. 184, 365–379 (2015).
Dobson, J. Sharks, wildlife tourism, and state regulation. Tour. Mar. Environ. 3(1), 15–23 (2006).
Newsome, D. & Rodger, K. To feed or not to feed: A contentious issues in wildlife tourism. R. Zool. Soc. N. S. W. 255–270 (2008).
Newsome, D., Lewis, A. & Moncrieff, D. Impacts and risks associated with developing, but unsupervised, stingray tourism at Hamelin Bay, Western Australia. Int. J. Tour. Res. 6(5), 305–323 (2004).
Bejder, L., Samuels, A., Whitehead, H., Finn, H. & Allen, S. Impact assessment research: Use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185 (2009).
Meyer, C. G., Dale, J. J., Papastamatiou, Y. P., Whitney, N. M. & Holland, K. N. Seasonal cycles and long-term trends in abundance and species composition of sharks associated with cage diving ecotourism activities in Hawaii. Environ. Conserv. 36(2), 104–111 (2009).
Clua, E. E. Managing bite risk for divers in the context of shark feeding ecotourism: A case study from French Polynesia (Eastern Pacific). Tour. Manag. 68, 275–283 (2018).
Gallagher, A. J. & Huveneers, C. P. Emerging challenges to shark-diving tourism. Mar. Policy 96, 9–12 (2018).
Thomson, J. A. et al. Feeding the world’s largest fish: Highly variable whale shark residency patterns at a provisioning site in the Philippines. R. Soc. Open Sci. 4(9), 170394 (2017).
Mourier, J., Claudet, J. & Planes, S. Human-induced shifts in habitat use and behaviour of a marine predator: The effects of bait provisioning in the blacktip reef shark. Anim. Conserv. 24(2), 230–238 (2021).
Lowe, C. G., Wetherbee, B. M. & Meyer, C. G. Using acoustic telemetry monitoring techniques to quantify movement patterns and site fidelity of sharks and giant trevally around French Frigate Shoals and Midway Atoll. Atoll Res. Bull. (2006).
Papastamatiou, Y. P., Itano, D. G., Dale, J. J., Meyer, C. G. & Holland, K. N. Site fidelity and movements of sharks associated with ocean-farming cages in Hawaii. Mar. Freshw. Res. 61(12), 1366–1375 (2010).
Clarke, C., Lea, J. S. E. & Ormond, R. F. G. Reef-use and residency patterns of a baited population of silky sharks, Carcharhinus falciformis, in the Red Sea. Mar. Freshw. Res. 62(6), 668–675 (2011).
Clua, E., Buray, N., Legendre, P., Mourier, J. & Planes, S. Behavioural response of sicklefin lemon sharks Negaprion acutidens to underwater feeding for ecotourism purposes. Mar. Ecol. Prog. Ser. 414, 257–266 (2010).
Brunnschweiler, J. M. & Barnett, A. Opportunistic visitors: Long-term behavioural response of bull sharks to food provisioning in Fiji. PLoS ONE 8(3), e58522 (2013).
Hammerschlag, N., Gallagher, A. J., Wester, J., Luo, J. & Ault, J. S. Don’t bite the hand that feeds: Assessing ecological impacts of provisioning ecotourism on an apex marine predator. Funct. Ecol. 26(3), 567–576 (2012).
Brunnschweiler, J. M. & Baensch, H. Seasonal and long-term changes in relative abundance of bull sharks from a tourist shark feeding site in Fiji. PLoS ONE 6(1), e16597 (2011).
McPhee, D. Unprovoked shark bites: Are they becoming more prevalent?. Coast. Manag. 42(5), 478–492 (2014).
Meyer, C. G. et al. Habitat geography around Hawaii’s oceanic islands influences tiger shark (Galeocerdo cuvier) spatial behaviour and shark bite risk at ocean recreation sites. Sci. Rep. 8(1), 1–18 (2018).
Heithaus, M. R., Wirsing, A. J., Dill, L. M. & Heithaus, L. I. Long-term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia. Mar. Biol. 151(4), 1455–1461 (2007).
Meyer, C. G., Papastamatiou, Y. P. & Holland, K. N. A multiple instrument approach to quantifying the movement patterns and habitat use of tiger (Galeocerdo cuvier) and Galapagos sharks (Carcharhinus galapagensis) at French Frigate Shoals, Hawaii. Mar. Biol. 157(8), 1857–1868 (2010).
Holmes, B. J. et al. Tiger shark (Galeocerdo cuvier) movement patterns and habitat use determined by satellite tagging in eastern Australian waters. Mar. Biol. 161(11), 2645–2658 (2014).
Werry, J. M. et al. Reef-fidelity and migration of tiger sharks, Galeocerdo cuvier, across the Coral Sea. PLoS ONE 9(1), e83249 (2014).
Wirsing, A. J., Heithaus, M. R. & Dill, L. M. Tiger shark (Galeocerdo cuvier) abundance and growth in a subtropical embayment: Evidence from 7 years of standardized fishing effort. Mar. Biol. 149(4), 961–968 (2006).
Lea, J. S. et al. Repeated, long-distance migrations by a philopatric predator targeting highly contrasting ecosystems. Sci. Rep. 5(1), 1–11 (2015).
Payne, N. L. et al. Combining abundance and performance data reveals how temperature regulates coastal occurrences and activity of a roaming apex predator. Glob. Change Biol. 24(5), 1884–1893 (2018).
Lear, K. O. et al. Thermal performance responses in free-ranging elasmobranchs depend on habitat use and body size. Oecologia 191(4), 829–842 (2019).
Hammerschlag, N. et al. Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark (Galeocerdo cuvier). Glob. Change Biol. 28(6), 1990–2005 (2022).
Bègue, M., Clua, E., Siu, G. & Meyer, C. Prevalence, persistence and impacts of residual fishing hooks on tiger sharks. Fish. Res. 224, 105462 (2020).
Vianna, G. M., Meekan, M. G., Meeuwig, J. J. & Speed, C. W. Environmental influences on patterns of vertical movement and site fidelity of grey reef sharks (Carcharhinus amblyrhynchos) at aggregation sites. PLoS ONE 8(4), e60331 (2013).
Laroche, R. K., Kock, A. A., Dill, L. M. & Oosthuizen, W. H. Effects of provisioning ecotourism activity on the behaviour of white sharks Carcharodon carcharias. Mar. Ecol. Prog. Ser. 338, 199–209 (2007).
Becerril-García, E. E., Hoyos-Padilla, E. M., Micarelli, P., Galván-Magaña, F. & Sperone, E. The surface behaviour of white sharks during ecotourism: A baseline for monitoring this threatened species around Guadalupe Island, Mexico. Aquat. Conserv. Mar. Freshwat. Ecosyst. 29(5), 773–782 (2019).
Becerril-García, E. E., Hoyos-Padilla, E. M., Micarelli, P., Galván-Magaña, F. & Sperone, E. Behavioural responses of white sharks to specific baits during cage diving ecotourism. Sci. Rep. 10(1), 1–11 (2020).
Holland, K. N., Wetherbee, B. M., Lowe, C. G. & Meyer, C. G. Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar. Biol. 134(4), 665–673 (1999).
Papastamatiou, Y. P. et al. Telemetry and random-walk models reveal complex patterns of partial migration in a large marine predator. Ecology 94(11), 2595–2606 (2013).
Matich, P., Heithaus, M. R. & Layman, C. A. Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J. Anim. Ecol. 80(1), 294–305 (2011).
Sperone, E. et al. Social interactions among bait-attracted white sharks at Dyer Island (South Africa). Mar. Biol. Res. 6(4), 408–414 (2010).
Clua, E., Chauvet, C., Read, T., Werry, J. M. & Lee, S. Y. Behavioural patterns of a tiger shark (Galeocerdo cuvier) feeding aggregation at a blue whale carcass in Prony Bay, New Caledonia. Mar. Freshw. Behav. Physiol. 46(1), 1–20 (2013).
Rangel, B. S. et al. Physiological state predicts space use of sharks at a tourism provisioning site. Anim. Behav. 191, 149–163 (2022).
Maljković, A. & Côté, I. M. Effects of tourism-related provisioning on the trophic signatures and movement patterns of an apex predator, the Caribbean reef shark. Biol. Cons. 144(2), 859–865 (2011).
Hammerschlag, N., Gutowsky, L. F. G., Gallagher, A. J., Matich, P. & Cooke, S. J. Diel habitat use patterns of a marine apex predator (tiger shark, Galeocerdo cuvier) at a high use area exposed to dive tourism. J. Exp. Mar. Biol. Ecol. 495, 24–34 (2017).
Sulikowski, J. A. et al. Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site. Aquat. Biol. 24(3), 175–184 (2016).
Hammerschlag, N. et al. Evaluating the landscape of fear between apex predatory sharks and mobile sea turtles across a large dynamic seascape. Ecology 96(8), 2117–2126 (2015).
Orams, M. B. Feeding wildlife as a tourism attraction: a review of issues and impacts. Tour. Manag. 23(3), 281–293 (2002).
Howard, P. & Jones, D. N. A qualitative study of wildlife feeding in south-east Queensland. In Urban Wildlife: More Than Meets the Eye (Eds Lunney, D. & Burgin, S.) 55–62 (2004).
Walpole, M. J. Feeding dragons in Komodo National Park: A tourism tool with conservation complications. Anim. Conserv. Forum 4(1), 67–73 (2001).
Aggimarangsee, N. Survey for semi-tame colonies of macaques in Thailand. Nat. Hist. Bull. Siam. Soc. 40, 103–166 (1993).
Heinrich, D. D., Huveneers, C., Houslay, T. M., Dhellemmes, F. & Brown, C. Shark habituation to a food-related olfactory cue. Anim. Behav. 187, 147–165 (2022).
Abrantes, K. G., Brunnschweiler, J. M. & Barnett, A. You are what you eat: Examining the effects of provisioning tourism on shark diets. Biol. Cons. 224, 300–308 (2018).
Heinrich, D. et al. Short-term impacts of daily feeding on the residency, distribution and energy expenditure of sharks. Anim. Behav. 172, 55–71 (2021).
Cisneros-Montemayor, A. M., Barnes-Mauthe, M., Al-Abdulrazzak, D., Navarro-Holm, E. & Sumaila, U. R. Global economic value of shark ecotourism: Implications for conservation. Oryx 47(3), 381–388 (2013).
Apps, K., Dimmock, K. & Huveneers, C. Turning wildlife experiences into conservation action: Can white shark cage-dive tourism influence conservation behaviour?. Mar. Policy 88, 108–115 (2018).
Zimmerhackel, J. S. et al. How shark conservation in the Maldives affects demand for dive tourism. Tour. Manag. 69, 263–271 (2018).
Clua, E. E. & Torrente, F. Determining the role of hand feeding practices in accidental shark bites on scuba divers. J. Forensic Sci. Criminol. 3(5), 502 (2015).
Healy, T. J., Hill, N. J., Barnett, A. & Chin, A. A global review of elasmobranch tourism activities, management and risk. Mar. Policy 118, 103964 (2020).
Catlin, J., Jones, T. & Jones, R. Balancing commercial and environmental needs: Licensing as a means of managing whale shark tourism on Ningaloo reef. J. Sustain. Tour. 20(2), 163–178 (2012).
Techera, E. J. & Klein, N. The role of law in shark-based eco-tourism: Lessons from Australia. Mar. Policy 39, 21–28 (2013).
Smith, K. R., Scarpaci, C., Scarr, M. J. & Otway, N. M. Scuba diving tourism with critically endangered grey nurse sharks (Carcharias taurus) off eastern Australia: Tourist demographics, shark behaviour and diver compliance. Tour. Manag. 45, 211–225 (2014).
Lagouy, E. & Clua, E. L’écotourisme animalier en Polynésie Française. Rapport final pour l’Agence des Aires Marines Protégées (Final report for the French Biodiversity Agency, Ministry of the Environment) (2016).
Durban, J. W. & Parsons, K. M. Laser-metrics of free-ranging killer whales. Mar. Mamm. Sci. 22, 735–743 (2006).
van Aswegen, M. et al. Morphological differences between coastal bottlenose dolphin (Tursiops aduncus) populations identified using non-invasive stereo-laser photogrammetry. Sci. Rep. 9(1), 1–14 (2019).
Whitney, N. M. & Crow, G. L. Reproductive biology of the tiger shark (Galeocerdo cuvier) in Hawaii. Mar. Biol. 151(1), 63–70 (2007).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/ (2022).
Author information
Authors and Affiliations
Contributions
E.C. designed the experiment. M.B. collected the data. C.S., J.M., C.M. & E.C. performed analysis. C.S. and C.M. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Séguigne, C., Bègue, M., Meyer, C. et al. Provisioning ecotourism does not increase tiger shark site fidelity. Sci Rep 13, 7785 (2023). https://doi.org/10.1038/s41598-023-34446-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34446-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.