Abstract
Metabolic reprogramming of cancer cells is a potential target for cancer therapy. It is also known that a hypoxic environment, one of the tumor microenvironments, can alter the energy metabolism from oxidative phosphorylation to glycolysis. However, the relationship between hypoxia and drug sensitivity, which targets energy metabolism, is not well known. In this study, A549 cells, a cell line derived from lung adenocarcinoma, were evaluated under normoxia and hypoxia for the sensitivity of reagents targeting oxidative phosphorylation (metformin) and glycolysis (α-cyano-4-hydroxycinnamic acid [CHC]). The results showed that a hypoxic environment increased the expression levels of monocarboxylate transporter (MCT) 4 and hypoxia-induced factor-1α (HIF-1α), whereas MCT1 and MCT2 expression did not vary between normoxia and hypoxia. Furthermore, the evaluation of the ATP production ratio indicated that glycolysis was enhanced under hypoxic conditions. It was then found that the sensitivity to metformin decreased while that to CHC increased under hypoxia. To elucidate this mechanism, MCT4 and HIF-1α were knocked down and the expression level of MCT4 was significantly decreased under both conditions. In contrast, the expression of HIF-1α was decreased by HIF-1α knockdown and increased by MCT4 knockdown. In addition, changes in metformin and CHC sensitivity under hypoxia were eliminated by the knockdown of MCT4 and HIF-1α, suggesting that MCT4 is involved in the phenomenon described above. In conclusion, it was shown that the sensitivity of reagents targeting energy metabolism is dependent on their microenvironment. As MCT4 is involved in some of these mechanisms, we hypothesized that MCT4 could be an important target molecule for cancer therapy.
Similar content being viewed by others
Introduction
Metabolic reprogramming is recognized as one of the hallmarks of cancer and is a target for cancer therapy1. Although aerobic glycolysis, known as the Warburg effect, is considered the characteristic metabolic state of cancer cells2, the reverse Warburg effect, in which oxidative cancer cells use lactate produced by glycolysis of surrounding fibroblasts as fuel for oxidative phosphorylation (OXPHOS), has recently been proposed as another metabolic characteristic of cancer cells3. Similarly, the metabolic response from OXPHOS to glycolysis in hypoxic environments in cancer cells is mediated by the stabilization of hypoxia-induced factor-1α (HIF-1α), which is an important factor in cancer metabolism and therapeutic targets4. However, the effects of hypoxic environment on drug sensitivity to energy metabolism remain unclear.
Metformin, an antidiabetic drug, has been shown to have anticancer effects5. The detailed mechanism of its anticancer activity is not clear, but it has various actions, one of which is the suppressive effects on OXPHOS mediated by the inhibition of mitochondrial respiratory chain complex I6. In a previous study, though metformin showed potent antitumor effects on those cancer cells that obtain energy from OXPHOS, its inhibitory effects were reduced in cells related to aerobic glycolysis7. This suggests that the sensitivity of oxidative-glycolysis cancer cells to these inhibitors is dependent on the metabolic performance of cancer cells.
Lactate, a metabolite of glycolysis and a source of energy for oxidative cancer cells, is a significant molecule, and monocarboxylate transporters (MCTs) transfer lactate across the plasma membrane into and out of the cell8. MCTs belong to the SLC16 family and consist of 14 isoforms9. Among them, MCT1, MCT2, and MCT4 are expressed in cancer cells, and high MCT4 expression indicates poor survival in various cancer types10. MCT1 and MCT2 are thought to contribute to lactate uptake by oxidative cancer cells, whereas MCT4 is thought to contribute to the extracellular release of lactate produced by glycolysis11. In addition, MCT4 is highly expressed in glycolytic cancer cells and supports their proliferation12. Moreover, MCTs are involved in Warburg and reverse Warburg effects3,13 and thus, aid in adaptation to hypoxic environment14. Consequently, MCT inhibitors are candidate anticancer drugs and several compounds in this regard have been reported15,16. α-Cyano-4-hydroxycinnamic acid (CHC), a classic and nonselective inhibitor of MCTs, has been shown to have antineoplastic effects and may accumulate lactate in cancer cells and suppress glycolysis17,18.
Considering this background, this study hypothesized that metabolic reprogramming from the OXPHOS preference phenotype to the glycolysis-dominant phenotype induced by a hypoxic environment would alter the antineoplastic effects of OXPHOS/glycolysis-targeting drugs. To test this hypothesis, A549 cells, a cell line derived from lung adenocarcinoma, were used because they show preference for OXPHOS metabolism compared with other lung adenocarcinoma cell lines7. Cells were cultured under normoxia or hypoxia, and their sensitivity to metformin as an OXPHOS inhibitor and CHC as a glycolysis inhibitor was evaluated.
Results
Hypoxia upregulated MCT4 and HIF-1α expression
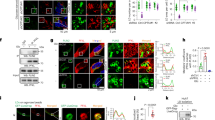
To confirm hypoxia-induced metabolic alterations, A549 cells were cultured under normoxia and hypoxia and the protein expression levels of MCT1, MCT2, MCT4, and HIF-1α (Fig. 1A, Supplementary Fig. 1) were evaluated. A significant increase in MCT4 and HIF-1α expression under hypoxia was observed compared to normoxia, while MCT1 and MCT2 expression did not differ between normoxia and hypoxia (Fig. 1B–E). In addition, an XF analyzer was used to measure the rate of glycolysis and oxidative ATP production (Fig. 2). Under normoxic conditions, A549 cells obtained ATP primarily via oxidative metabolism. In contrast, hypoxia changed the phenotype of A549 cells from oxidative to glycolytic (Table 1). Therefore, it was hypothesized that hypoxia induces metabolic changes from OXPHOS to glycolysis.
Protein expression level of MCT1, MCT2, MCT4, and HIF-1α in normoxia and hypoxia. (A) Western blot analysis of MCT1, MCT2, MCT4, and HIF-1α in normoxia and hypoxia in A549 cells. The protein expression levels of MCT1 (B), MCT2 (C), MCT4 (D), and HIF-1α (E) were visualized, respectively. Data are presented as mean ± standard error of three independent experiments. *p < 0.05 compared with the normoxia using unpaired Student’s t test.
ATP production rate in normoxia and hypoxia. Mitochondrial respiratory activity was evaluated using a Seahorse XFp analyzer. The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were calculated by normalizing the cell counts under normoxia (A) and hypoxia (B). Each mark represents the mean with a positive SE of three or four independent experiments. (C) ATP production rate under normoxia and hypoxia calculated from (A,B), respectively. Each mark represents the mean of three or four independent experiments.
Hypoxia attenuated the effects of OXPHOS-targeting inhibitors and enhanced the effects of glycolysis-targeting inhibitors
An MTT assay was used to determine the antitumor effects of the OXPHOS-targeting inhibitor metformin and glycolysis-targeting inhibitor CHC under various oxygen conditions (Fig. 3). The cell viability was decreased by metformin and CHC in a concentration-dependent manner under all oxygen conditions. Notably, the degree of anticancer effects induced by 10 mM metformin was reduced under hypoxia in a time-dependent manner (44.6%, 57.2%, and 73.2% under normoxia for 72 h, normoxia for 24 h + hypoxia for 48 h, and hypoxia for 72 h, respectively) (Fig. 3A–C). In contrast, the opposite results were observed with the addition of 5 mM CHC (85.4%, 77.3%, and 71.7% under normoxia for 72 h, normoxia for 24 h + hypoxia for 48 h, and hypoxia for 72 h, respectively) (Fig. 3D–F), which is consistent with the hypothesis that drug sensitivity varies in response to altered metabolic conditions in a hypoxic environment.
Effects of metformin and CHC on cell viability with concentration dependency of each reagent and time dependency of hypoxic dependency. Concentration dependence of metformin 10 mM and 20 mM (A–C) and CHC 5 mM and 10 mM (D–F) for cell viability using the MTT assay. Time dependence of cell viability under hypoxic conditions (normoxia 72 h, normoxia 24 h + hypoxia 48 h, and hypoxia 72 h) using the MTT assay. Data are presented as the mean ± standard error of three independent experiments. *p < 0.05, compared with the control; †p < 0.05, compared with metformin 10 mM or CHC 5 mM using the Tukey–Kramer test.
MCT4 expression was involved in the alteration of drug sensitivity in hypoxic conditions
Hypoxia increased the expression of MCT4 and HIF-1α and altered energy metabolism, which may induce changes in the sensitivity of metformin and CHC on A549 cells. Therefore, the reagent sensitivity of A549 cells transfected with MCT4 and HIF-1α siRNAs was examined. Western blotting analysis showed that the expression of HIF-1α was decreased by HIF-1α siRNA and increased by MCT4 siRNA, particularly under hypoxia (Fig. 4A,B, Supplementary Fig. 2). In contrast, MCT4 expression was decreased by all the siRNAs, with MCT4 siRNA#1 being the most potent (Supplementary Fig. 3). Consequently, HIF-1α positively regulates MCT4 expression.
Effects of MCT4 and HIF-1α siRNA on protein expression level and sensitivity of metformin and CHC. Western blot analysis after transfection with MCT4 and HIF-1α siRNA under normoxia (A) and hypoxia (B). Cell viability after treatment with 10 mM metformin under normoxia (C) and hypoxia (D) in A549 cells transfected with siRNA. Viability of CHC 5 mM under normoxia (E) and hypoxia (F) in A549 cells transfected with siRNA. Data are presented as the mean ± standard error of three independent experiments. *p < 0.05, compared with the control; †p < 0.05, compared with metformin 10 mM or CHC 5 mM using the Tukey–Kramer test.
Finally, the effects of metformin and CHC on the viability of the siRNA-transfected A549 cells were evaluated. In normoxia, knockdown of MCT4 and HIF-1α had no impact on the sensitivity to metformin or CHC (Fig. 4C,E). Interestingly, the sensitivity of A549 cells to metformin under hypoxic conditions increased significantly with MCT4 siRNA #1 treatment (Fig. 4D). Moreover, CHC sensitivity significantly decreased in A549 cells transfected with MCT4 siRNA#1 and HIF-1α siRNA (Fig. 4F). Therefore, MCT4 expression may be associated with hypoxia-induced alterations in drug sensitivity.
Discussion
Metabolic reprogramming including MCTs that are involved in lactate shuttling between glycolytic and oxidative cancer cells, is a new target for cancer therapy. The microenvironment (for e.g., hypoxia) of tumor cells, is also an important factor in the energetic metabolism of cancer cells. This experiment demonstrated the hypoxia-induced enhancement of MCT4 and HIF-1α protein expression (Fig. 1A,D,E). In addition, knockdown experiments showed that HIF-1α positively regulates MCT4 expression. These results are in agreement with those of the previous studies4,19. In addition, the ATP production ratio of A549 cells was converted from OXPHOS to glycolysis under hypoxia (Fig. 2), which indicated that hypoxia directly induced metabolic alterations. Furthermore, sensitivity to metformin was attenuated and sensitivity to CHC was enhanced by hypoxia. These results are also coordinated with the inhibition targets of these reagents. In terms of the effects of siRNA, a decrease in MCT4 expression did not change reagent sensitivity under normoxic conditions, which indicated that MCT4 does not function to release extracellular lactate in normoxia because pyruvate and lactate are poorly produced by glycolysis and are consumed by OXPHOS. In contrast, under hypoxia, MCT4 contributes to the release of lactate accumulated by glycolysis and regulates the alteration of energy metabolism. Therefore, MCT4 could counteract the variability in metformin and CHC sensitivity in hypoxia, and may play an important role in the response to hypoxia and the alteration of energy metabolism. Based on these results, we concluded that MCT4 could be a therapeutic target for cancer via energy metabolism.
Metformin inhibits OXPHOS and exerts antineoplastic effects. In the present study, the efficacy of metformin was attenuated under hypoxic conditions (Fig. 3A–C). Previous studies on metformin sensitivity in various lung cancer cell lines have shown that this effect is more pronounced in cell lines with predominant OXPHOS7. Similarly, in colorectal cancer, the sensitivity to metformin varies depending on the metabolic state of the cells, and it has been confirmed that metformin has a weak effect on phenotypes in which the glycolytic system is dominant20. In addition, low glucose levels increased the sensitivity to metformin, and this phenomenon was more pronounced in cells with predominant OXPHOS21. These phenomena seem reasonable because energy production under OXPHOS at low glucose levels is more dominant than that in the glycolytic system. In contrast, the present study evaluated the changes from OXPHOS to glycolysis induced by hypoxia. Other studies on metformin sensitivity have reported that mitochondrial function was enhanced in cisplatin-resistant cell lines, and metformin-induced inhibition of oxidative phosphorylation was improved in these resistant cells22. Two other enzymes important for cancer metabolism are lactate dehydrogenase (LDH) A and LDH B, which catalyze the formation of pyruvate to lactate, and the reverse reaction, respectively23. LDHA/B double knockout causes a shift to OXPHOS, low proliferation in hypoxia, and sensitization to phenformin, another mitochondrial complex I inhibitor24. Thus, the inhibition of OXPHOS by metformin is more effective in cells with a predominance of OXPHOS, suggesting that the intensity of the effect of metformin varies depending on the metabolic state of cancer cells. Metformin has been reported to improve progression-free survival in meta-analyses of advanced lung cancer25. In contrast, a randomized controlled trial indicated that metformin lowered the survival outcomes of patients with locally advanced NSCLC26. These clinical results also suggest that the efficacy of metformin depends on the cancer type and staging.
Since MCT1, MCT2, and MCT4 are highly expressed in cancer cells, inhibitors of these are potential anticancer drugs. AZD3965, a dual inhibitor of MCT1 and MCT2, is at phase I trial for advanced cancer. In a previous study using small cell lung cancer cell lines, it was reported that sensitivity to AZD3965 varied between cell lines27. In addition, cell lines that did not express MCT4 showed increasing sensitivity to MCT1 inhibition by AZD3965 under hypoxia; whereas cell lines that expressed MCT4, whose expression increased under hypoxia showed resistance to AZD396527. In addition, MCT4 expression abolished glycolysis suppression induced by AR-C155858, another MCT1/2 dual inhibitor, and attenuated the antitumor effects of AR-C15585828. Taken together with the results of this study, it is possible that MCT1 is involved in the response of energy metabolism to hypoxia in cells that do not express MCT4, and that cells are therefore more sensitive to its inhibitors. However, in cells that express MCT4, MCT4 responds to hypoxia and the effects of MCT1 inhibition are no longer observed. Furthermore, Noble et al. (2017) reported that the combination of AZD3965 and metformin caused significant cell death in a Hodgkin’s lymphoma cell line in which MCT1 was predominantly expressed29, which is appropriate because the energy derived from glycolysis and OXPHOS was significantly inhibited. A similar phenomenon has been observed in diffuse large B-cell lymphoma that lacks MCT4 expression, and the simultaneous targeting of glycolysis and oxidative phosphorylation by the combination of AZD3965 and IACS-010759, an inhibitor of mitochondrial complex I, was recently reported to be more effective30. Furthermore, Epstein-Barr virus (EBV) infection promotes B lymphoid tumors, and EBV-infected B cells upregulate MCT4, resulting in resistance to AZD396531. In this case, VB124, an MCT4 inhibitor, had antitumor effect, and the combination of AZD3965 and VB124 sensitized the killing by metformin31. Consequently, the simultaneous inhibition of MCTs and mitochondrial respiration has strong fatal effects against tumor cells32. This study revealed that the classical MCTs non-selective inhibitor CHC suppressed the viability of A549 cells. The sensitivity of CHC increased under hypoxia with the upregulation of MCT4, which indicated that MCT4 was involved in the alteration of energy metabolism induced by hypoxia.
In conclusion, we demonstrated that hypoxia induced metabolic alterations from OXPHOS to glycolysis and altered the sensitivity of metabolic targeting inhibitors. MCT4 is partially involved in the mechanisms underlying these phenomena. To the best of our knowledge, this is the first study to reveal the association between oxygen conditions and drug sensitivity in inhibiting energy metabolism. These results will contribute to cancer therapies that target MCT4. However, this study has a major limitation as for reproducibility because we performed experiment only on A549 cell line. In this paper, we used normoxic and hypoxic A549 cell line to compare the drug sensitivity in oxygen states. Similarly, A549 cell line was used to evaluate glycolysis and OXPHOS under various conditions in recent articles33,34. In addition, the energy metabolic adaptation to environmental condition were similar in multiple cancer cells including A549 cells35. Furthermore, A549 cell line was one of the most common used lung cancer cells and was evaluated for the regulation of MCTs expression36,37. Therefore, we believe that our report is valuable. Although the metabolic characteristics of A549 cell line are identified among other lung cancer cell lines7, further research using other cancer cell lines is required to substantiate our findings.
Materials and methods
Chemicals
Metformin was purchased from Enzo Biochem (New York, NY, USA) and CHC was purchased from Sigma-Aldrich (St. Louis, MO, USA). All the compounds used were of the highest purity available.
Cell culture
A549 cells (American Type Culture Collection, Manassas, VA, USA) were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C in a 5% CO2 atmosphere under normoxia and hypoxia (5% O2). A BIONEZ-3 hypoxia culture kit (Sugiyamagen, Tokyo, Japan) was used to determine the hypoxic environment.
Western blotting
Cells were lysed in a lysis buffer supplemented with 60 mM Tris–HCl (pH 6.8) and 1% sodium dodecyl sulfate (SDS) in distilled water. The lysate was stored on ice for 5 min and sonicated briefly at 4 °C. The sample was centrifuged at 12,000×g for 15 min at 4 °C and the clear supernatant was collected. The protein concentration was determined using a Pierce® bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific). All lysates were added to loading buffer containing 0.1 M tris-hydrochloride, 4% SDS, 10% 2-mercaptoethanol, 20% glycerol, and 0.004% bromophenol blue. Proteins (40 μg) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad). The membranes were blocked with PBS containing 0.05% Tween 20 (PBS/T) and 1% non-fat dry milk for 1 h at room temperature. After washing with PBS/T, the membranes were incubated overnight at 4 °C with primary antibodies against MCT1 (1:200, SC-365501, Santa Cruz Biotechnology), MCT2 (1:1000, ab198272, Abcam), MCT4 (1:1000, 22787-1-AP, Proteintech), HIF-1α (1:200, sc-10790, Santa Cruz Biotechnology), and actin (1:1000, ab179467, Abcam). The membranes were cut prior to hybridization with antibodies in HIF-1α and actin. The bands were detected using horseradish peroxidase (HRP)-conjugated secondary antibodies (1:4000) and visualized using ECL Western Blotting Detection Reagents (Cytiva).
Measure of ECAR and OCR
The extracellular flux analyzer provided a continuous quantitative assessment of mitochondrial respiration, as previously reported38,39,40. Seahorse XFp (Agilent Technologies, Santa Clara, CA, USA) was used to measure the mitochondrial oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of A549 cells using a Seahorse XF Real-Time ATP Rate Assay Kit. These parameters were estimated by the sequential addition of oligomycin (an inhibitor of ATP synthesis), rotenone, and antimycin A (complete inhibition of mitochondrial respiration), and the OCRs and ECARs were measured.
At 48 h before the start of the experiment, 10,000 cells per well were seeded into 8-well plates and incubated at 37 °C under an atmosphere of 5% CO2 under normoxia and hypoxia (5% O2). Prior to analysis, the cells were incubated in analytical medium at 37 °C and CO2-free for 1 h. After measuring the baseline OCRs (pmol/min) and ECARs (mpH/min), oligomycin (final concentration of 2 μM), rotenone (final concentration, 0.5 μM), and antimycin A (final concentration of 0.5 μM) were continuously added to the cells. The obtained data (Fig. 2A,B) were used to determine the ATP production rate (pmol/min/cell) (Fig. 2C, Table 1).
MTT assay
Cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 24 h after seeding, various concentrations of metformin and CHC were added and incubated for 48 h. The MTT solution was added to the medium, and the cells were further incubated for 1 h. The MTT medium was then replaced with dimethyl sulfoxide (DMSO), and the absorbance was measured at 590 nm.
RNA interference
The siRNA transfection was optimized using Lipofectamine RNAiMAX (Invitrogen). Silencer Select siRNA against MCT4 (#1; s17416 and #2; 117233), HIF-1α (42840), and negative control siRNA (Silencer Negative Control No. 1 siRNA) were purchased from Thermo Fisher Scientific. A549 cells were transfected with siRNA via reverse transfection at a final concentration of 10 nM. After siRNA transfection (24 h), cells were used for various experiments after 48 h.
Statistical analysis
Statistical analyses of the data were performed using the unpaired Student’s t test or Tukey–Kramer test. Data were analyzed using SigmaPlot 14.5, and differences were considered statistically significant at p < 0.05.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yoshida, G. J. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J. Exp. Clin. Cancer Res. 34, 111 (2015).
Icard, P. et al. How the Warburg effect supports aggressiveness and drug resistance of cancer cells?. Drug Resist. Updates 38, 1–11 (2018).
Wilde, L. et al. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 44, 198–203 (2017).
Rosafio, K. & Pellerin, L. Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1α-mediated transcriptional regulation. Glia 62, 477–490 (2014).
Morales, D. R. & Morris, A. D. Metformin in cancer treatment and prevention. Annu. Rev. Med. 66, 17–29 (2014).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3, e02242 (2014).
Kuo, T. C. et al. Monocarboxylate transporter 4 is a therapeutic target in non-small cell lung cancer with aerobic glycolysis preference. Mol. Ther. Oncolytics 18, 189–201 (2020).
Kobayashi, M., Narumi, K., Furugen, A. & Iseki, K. Transport function, regulation, and biology of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4). Pharmacol. Ther. 226, 107862 (2021).
Halestrap, A. P. & Wilson, M. C. The monocarboxylate transporter family—Role and regulation. IUBMB Life 64, 109–119 (2012).
Bovenzi, C. D. et al. Prognostic indications of elevated MCT4 and CD147 across cancer types: A meta-analysis. Biomed Res. Int. 2015, 242437 (2015).
Contreras-Baeza, Y. et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 294, 20135–20147 (2019).
Payen, V. L., Mina, E., Van Hée, V. F., Porporato, P. E. & Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 33, 48–66 (2020).
Jiang, X., Yan, N., Deng, D. & Yan, C. Structural aspects of the glucose and monocarboxylate transporters involved in the Warburg effect. IUBMB Life 74, 1180–1199 (2022).
Marchiq, I. & Pouysségur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H(+) symporters. J. Mol. Med. (Berl) 94, 155–171 (2016).
Wang, N. et al. Structural basis of human monocarboxylate transporter 1 inhibition by anti-cancer drug candidates. Cell 184, 370-383.e13 (2021).
Futagi, Y., Kobayashi, M., Narumi, K., Furugen, A. & Iseki, K. Identification of a selective inhibitor of human monocarboxylate transporter 4. Biochem. Biophys. Res. Commun. 495, 427–432 (2018).
Granja, S. et al. The monocarboxylate transporter inhibitor α-cyano-4-hydroxycinnamic acid disrupts rat lung branching. Cell Physiol. Biochem. 32, 1845–1856 (2013).
Guan, X. & Morris, M. E. In vitro and in vivo efficacy of AZD3965 and alpha-cyano-4-hydroxycinnamic acid in the Murine 4T1 breast tumor model. AAPS J. 22, 84 (2020).
Nadai, T. et al. Pharmacological inhibition of MCT4 reduces 4-hydroxytamoxifen sensitivity by increasing HIF-1α protein expression in ER-positive MCF-7 breast cancer cells. Biol. Pharm. Bull. 44, 1247–1253 (2021).
Alhourani, A. H. et al. Metformin treatment response is dependent on glucose growth conditions and metabolic phenotype in colorectal cancer cells. Sci. Rep. 11, 10487 (2021).
Zhuang, Y., Chan, D. K., Haugrud, A. B. & Miskimins, W. K. Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo. PLoS ONE 9, e108444 (2014).
Cruz-Bermúdez, A. et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic. Biol. Med. 135, 167–181 (2019).
Pouysségur, J. et al. “Warburg effect” controls tumor growth, bacterial, viral infections and immunity—Genetic deconstruction and therapeutic perspectives. Semin. Cancer Biol. 86, 334–346 (2022).
Ždralević, M. et al. Double genetic disruption of lactate dehydrogenases A and B is required to ablate the “Warburg effect” restricting tumor growth to oxidative metabolism. J. Biol. Chem. 293, 15947–15961 (2018).
Luo, X., Chen, X., Wang, L., Yang, B. & Cai, S. Metformin adjunct with antineoplastic agents for the treatment of lung cancer: A meta-analysis of randomized controlled trials and observational cohort studies. Front. Pharmacol. 12, 639016 (2021).
Tsakiridis, T. et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: The OCOG-ALMERA randomized clinical trial. JAMA Oncol. 7, 1333–1341 (2021).
Polański, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Le Floch, R. et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 108, 16663–16668 (2011).
Noble, R. A. et al. Inhibition of monocarboxyate transporter 1 by AZD3965 as a novel therapeutic approach for diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica 102, 1247–1257 (2017).
Noble, R. A. et al. Simultaneous targeting of glycolysis and oxidative phosphorylation as a therapeutic strategy to treat diffuse large B-cell lymphoma. Br. J. Cancer 127, 937–947 (2022).
Bonglack, E. N. et al. Monocarboxylate transporter antagonism reveals metabolic vulnerabilities of viral-driven lymphomas. Proc. Natl. Acad. Sci. USA 118, e2022495118 (2021).
Benjamin, D. & Hall, M. N. Combining metformin with lactate transport inhibitors as a treatment modality for cancer—Recommendation proposal. Front. Oncol. 12, 1034397 (2022).
Öğünç Keçeci, Y. & İncesu, Z. Mitochondrial oxidative phosphorylation became functional under aglycemic hypoxia conditions in A549 cells. Mol. Biol. Rep. 49, 8219–8228 (2022).
Prusinkiewicz, M. A. et al. Differential effects of human adenovirus E1A protein isoforms on aerobic glycolysis in A549 human lung epithelial cells. Viruses 12, 610 (2020).
Shiratori, R. et al. Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci. Rep. 10, 18699 (2019).
Huang, H. K. et al. Isoorientin decreases cell migration via decreasing functional activity and molecular expression of proton-linked monocarboxylate transporters in human lung cancer cells. Am. J. Chin. Med. 48, 201–222 (2020).
Takenaga, K. et al. MCT4 is induced by metastasis-enhancing pathogenic mitochondrial NADH dehydrogenase gene mutations and can be a therapeutic target. Sci. Rep. 25, 13302 (2021).
Sasaki, D., Abe, J., Takeda, A., Harashima, H. & Yamada, Y. Transplantation of MITO cells, mitochondria activated cardiac progenitor cells, to the ischemic myocardium of mouse enhances the therapeutic effect. Sci. Rep. 12, 4344 (2022).
Tsujioka, T., Sasaki, D., Takeda, A., Harashima, H. & Yamada, Y. Resveratrol-encapsulated mitochondria-targeting liposome enhances mitochondrial respiratory capacity in myocardial cells. Int. J. Mol. Sci. 23, 112 (2021).
Yamada, Y., Somiya, K., Miyauchi, A., Osaka, H. & Harashima, H. Validation of a mitochondrial RNA therapeutic strategy using fibroblasts from a Leigh syndrome patient with a mutation in the mitochondrial ND3 gene. Sci. Rep. 10, 7511 (2020).
Acknowledgements
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 21H0423201) (provided to AY) and KAKENHI (Grant Number 20K07171) (provided to MK). We would like to thank Editage (http://www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
A.Y. designed the experiments and wrote the first draft of the manuscript. A.Y. and Y.Y. conducted experiments. A.Y., Y.Y., and M.K. analyzed the results. M.K., Y.M., T.S., K.N., and A.F. contributed to writing the manuscript. All authors have reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamaguchi, A., Mukai, Y., Sakuma, T. et al. Monocarboxylate transporter 4 involves in energy metabolism and drug sensitivity in hypoxia. Sci Rep 13, 1501 (2023). https://doi.org/10.1038/s41598-023-28558-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-28558-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.