Abstract
Gasdermin D (GSDMD) is the common effector for cytokine secretion and pyroptosis downstream of inflammasome activation and was previously shown to form large transmembrane pores after cleavage by inflammatory caspases to generate the GSDMD N-terminal domain (GSDMD-NT)1,2,3,4,5,6,7,8,9,10. Here we report that GSDMD Cys191 is S-palmitoylated and that palmitoylation is required for pore formation. S-palmitoylation, which does not affect GSDMD cleavage, is augmented by mitochondria-generated reactive oxygen species (ROS). Cleavage-deficient GSDMD (D275A) is also palmitoylated after inflammasome stimulation or treatment with ROS activators and causes pyroptosis, although less efficiently than palmitoylated GSDMD-NT. Palmitoylated, but not unpalmitoylated, full-length GSDMD induces liposome leakage and forms a pore similar in structure to GSDMD-NT pores shown by cryogenic electron microscopy. ZDHHC5 and ZDHHC9 are the major palmitoyltransferases that mediate GSDMD palmitoylation, and their expression is upregulated by inflammasome activation and ROS. The other human gasdermins are also palmitoylated at their N termini. These data challenge the concept that cleavage is the only trigger for GSDMD activation. They suggest that reversible palmitoylation is a checkpoint for pore formation by both GSDMD-NT and intact GSDMD that functions as a general switch for the activation of this pore-forming family.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The RNA-seq data supporting the findings of this study have been deposited at Sequence Read Archive BioProject accession PRJNA1054142 (corresponding to data in Supplementary Fig. 5c–e). The cryo-EM density maps of full-length GSDMD and the 33-fold symmetric full-length GSDMD pore have been deposited at the Electron Microscopy Data Bank under accession numbers EMD-44035 and EMD-44034, respectively. All other data are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Kayagaki, N. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Liu, X. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016).
Ding, J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016).
Gaidt, M. M. & Hornung, V. The NLRP3 inflammasome renders cell death pro-inflammatory. J. Mol. Biol. 430, 133–141 (2018).
Broz, P. & Dixit, V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Ruan, J., Xia, S., Liu, X., Lieberman, J. & Wu, H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018).
Xia, S. et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 20, 384–405 (2021).
Shi, J., Gao, W. & Shao, F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017).
Deng, W. et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 (2022).
Liu, Z. et al. Crystal structures of the full-length murine and human gasdermin d reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49 (2019).
Jiang, H. et al. Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem. Rev. 118, 919–988 (2018).
Mesquita, F. S. et al. Mechanisms and functions of protein S-acylation. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-024-00700-8 (2024).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Sobocinska, J., Roszczenko-Jasinska, P., Ciesielska, A. & Kwiatkowska, K. Protein palmitoylation and its role in bacterial and viral infections. Front. Immunol. 8, 2003 (2017).
Davda, D. et al. Profiling targets of the irreversible palmitoylation inhibitor 2-bromopalmitate. ACS Chem. Biol. 8, 1912–1917 (2013).
Xie, Y. et al. GPS-Lipid: a robust tool for the prediction of multiple lipid modification sites. Sci. Rep. 6, 28249 (2016).
Hu, L. et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 11, 281 (2020).
Aglietti, R. A. et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. USA 113, 7858–7863 (2016).
Sorek, N. & Yalovsky, S. Analysis of protein S-acylation by gas chromatography-coupled mass spectrometry using purified proteins. Nat. Protoc. 5, 834–840 (2010).
Ji, Y. et al. Direct detection of S-palmitoylation by mass spectrometry. Anal. Chem. 85, 11952–11959 (2013).
Devant, P. et al. Gasdermin D pore-forming activity is redox-sensitive. Cell Rep. 42, 112008 (2023).
Evavold, C. L. et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 184, 4495–4511 (2021).
Johnson, A. G. et al. Bacterial gasdermins reveal an ancient mechanism of cell death. Science 375, 221–225 (2022).
Gritsenko, A. et al. Priming is dispensable for NLRP3 inflammasome activation in human monocytes in vitro. Front. Immunol. 11, 565924 (2020).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Zhuang, Z., Gu, J., Li, B. O. & Yang, L. Inhibition of gasdermin D palmitoylation by disulfiram is crucial for the treatment of myocardial infarction. Transl. Res. 264, 66–75 (2024).
Saurin, A. T., Neubert, H., Brennan, J. P. & Eaton, P. Widespread sulfenic acid formation in tissues in response to hydrogen peroxide. Proc. Natl Acad. Sci. USA 101, 17982–17987 (2004).
Munoz-Planillo, R. et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Zhang, P. et al. NLRC4 inflammasome-dependent cell death occurs by a complementary series of three death pathways and determines lethality in mice. Sci. Adv. 7, eabi9471 (2021).
Miao, R. et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 56, 2523–2541 (2023).
Chen, D., Zhang, X. Y. & Shi, Y. Identification and functional characterization of hCLS1, a human cardiolipin synthase localized in mitochondria. Biochem. J. 398, 169–176 (2006).
Horvath, S. E. & Daum, G. Lipids of mitochondria. Prog. Lipid Res. 52, 590–614 (2013).
Dudek, J. Role of cardiolipin in mitochondrial signaling pathways. Front. Cell Dev. Biol. 5, 90 (2017).
Chu, C. T. et al. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205 (2013).
de Kroon, A. I., Dolis, D., Mayer, A., Lill, R. & de Kruijff, B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta 1325, 108–116 (1997).
Liu, J. et al. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol. Cancer Res. 1, 892–902 (2003).
Zhou, X. et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat. Genet. 54, 1305–1319 (2022).
Lanigan, T. M. et al. Real time visualization of cancer cell death, survival and proliferation using fluorochrome-transfected cells in an IncuCyte((R)) imaging system. J Biol Methods 7, e133 (2020).
Puthenveetil, R. et al. S-acylation of SARS-CoV-2 spike protein: mechanistic dissection, in vitro reconstitution and role in viral infectivity. J. Biol. Chem. 297, 101112 (2021).
Ko, P. J. et al. A ZDHHC5-GOLGA7 protein acyltransferase complex promotes nonapoptotic cell death. Cell Chem. Biol. 26, 1716–1724 (2019).
Orre, L. M. et al. SubCellBarCode: proteome-wide mapping of protein localization and relocalization. Mol Cell 73, 166–182 (2019).
Hao, J. W. et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 11, 4765 (2020).
Shimell, J. J. et al. The X-linked intellectual disability gene Zdhhc9 Is essential for dendrite outgrowth and inhibitory synapse formation. Cell Rep. 29, 2422–2437 (2019).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, eaat2738 (2018).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Mesquita, F. S. et al. S-acylation controls SARS-CoV-2 membrane lipid organization and enhances infectivity. Dev. Cell 56, 2790–2807 (2021).
Percher, A., Thinon, E. & Hang, H. Mass-tag labeling using acyl-PEG exchange for the determination of endogenous protein S-fatty acylation. Curr. Protoc. Protein Sci. 89, 14.17.11–14.17.11 (2017).
Okondo, M. C. et al. Inhibition of Dpp8/9 activates the Nlrp1b inflammasome. Cell Chem. Biol. 25, 262–267 (2018).
Marim, F. M., Silveira, T. N., Lima, D. S. Jr & Zamboni, D. S. A method for generation of bone marrow-derived macrophages from cryopreserved mouse bone marrow cells. PLoS ONE 5, e15263 (2010).
Liu, Z. et al. Caspase-1 engages full-length gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity 53, 106–114 (2020).
Brigidi, G. S. & Bamji, S. X. Detection of protein palmitoylation in cultured hippocampal neurons by immunoprecipitation and acyl-biotin exchange (ABE). J. Vis. Exp. (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Sanjana, N. E., Shalem, O. & Zhang, F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods 11, 783–784 (2014).
Zhang, Y. et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell Biol. 20, 1181–1192 (2018).
Gault, J. et al. High-resolution mass spectrometry of small molecules bound to membrane proteins. Nat. Methods 13, 333–336 (2016).
Durbin, K. R. et al. ProSight native: defining protein complex composition from native top-down mass spectrometry data. J. Proteome Res. 22, 2660–2668 (2023).
Fornelli, L. et al. Accurate sequence analysis of a monoclonal antibody by top-down and middle-down orbitrap mass spectrometry applying multiple ion activation techniques. Anal. Chem. 90, 8421–8429 (2018).
von Moltke, J. et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 490, 107–111 (2012).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Goddard, T. D. et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 27, 14–25 (2018).
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Delano, W. L. The PyMol Molecular Graphics System. (Delano Scientific, 2002).
Baroni, L., Abreu-Filho, P. G., Pereira, L. M., Nagl, M. & Yatsuda, A. P. Recombinant actin-depolymerizing factor of the apicomplexan Neospora caninum (NcADF) is susceptible to oxidation. Front. Cell Infect. Microbiol. 12, 952720 (2022).
Roschitzki-Voser, H. et al. Human caspases in vitro: expression, purification and kinetic characterization. Protein Expr. Purif. 84, 236–246 (2012).
Acknowledgements
We thank D. Bachovchin for GSDMD-KO THP-1 cells, C. Serhan for suggestions for UPLC–MS/MS, the staff at Harvard Medical School Nikon Imaging Center for help with fluorescence microscopy and K. Durbin and the team at Proteinaceous for their ongoing assistance with TD Validator and Prosight Native. The graphical diagram was created using BioRender. This work was supported by US National Institutes of Health grants AI139914 (to H.W. and J.L.), HD087988 (to H.W.), AI124491 (to H.W.), CA240955 (to J.L.), AT010268 (to S.F.O.), AI167993 (to J.C.K.), AI116550 (to J.C.K.) and DK34854 (to J.C.K.). C.W. received a postdoctoral fellowship from NIGMS. P.D. was supported by a PhD Fellowship by the Boehringer Ingelheim Fonds. X.P. received a postdoctoral fellowship from the Cancer Research Institute. P.F. received a postdoctoral fellowship from the Cancer Research Institute. Y.D. received a postdoctoral fellowship from the Charles A. King Trust. T.J.E.-B. is an EP Abraham Junior Research Fellow at Linacre College. C.A.L. is a Research Fellow at Wolfson College.
Author information
Authors and Affiliations
Contributions
H.W., G.D., L.B.H., L.D., C.W. and J.L. conceived the study. G.D. designed and performed most of the experiments. L.B.H. designed and performed most of the remaining experiments. T.J.E.-B. and C.A.L. designed and performed intact MS under C.V.R.’s supervision. B. Goh and G.D. designed and performed UPLC–MS/MS analysis with S.F.O. and H.W.’s supervision. B. Gu performed CRISPR–Cas9 knockout of ZDHHC5 and ZDHHC9 under J.L.’s supervision. P.D. prepared primary BMDMs under J.C.K.’s supervision. A. Balasubramanian and H.R.L. provided siRNAs for knocking down ZDHHC genes. X.P. helped with cryo-EM data processing for full-length GSDMD pore structure. P.F. and Y.D. helped with GSDMD and CASP1 expression, respectively. R.M. provided Crls1- or Plscr3-KO iBMDMs. X.M. attempted knock-in of the Halo tag to GSDMD in THP-1 cells. R.P. and A. Banerjee provided Heatil screen constructs. H.W., L.B.H., G.D. and J.L. wrote the manuscript with input from all of the authors.
Corresponding authors
Ethics declarations
Competing interests
H.W. and J.L. are cofounders of Ventus Therapeutics. J.C.K. consults and holds equity in Corner Therapeutics, Larkspur Biosciences, MindImmune Therapeutics and Neumora Therapeutics. None of these relationships impacted this study. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Françoise van der Goot and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 GSDMD is palmitoylated at its N-terminus at Cys191.
a, Protein sequence of human GSDMD N-terminal domain (GSDMD-NT). Certain residues are marked by colours. b, Domain organization and ribbon diagrams of autoinhibited full-length (FL) GSDMD (modelled after PDB 6N9O, left), membrane-inserted GSDMD-NT monomer (middle), and the GSDMD-NT pore oligomer modelled after PDB 6VFE (right). c, GSDMD-FL palmitoylation detected by alkyne stearic acid and rhodamine azide, and its inhibition by the general palmitoylation inhibitor 2-BP. d, Click chemistry for protein N-myristoylation did not detect N-myristoylation of GSDMD using the alkyne-myristate 13-TDYA. e, GSDMD-FL palmitoylation when expressed in HEK293T cells detected by ABE, with the known palmitoylated protein Calnexin as a positive control. f, GSDMD-NT palmitoylation when expressed in HEK293T cells, detected by ABE, and its inhibition by 2-BP. GAPDH acts as the loading control. g-l, PI staining (g), PI positivity (h), LDH release (i), cell viability (j), anti-FLAG immunofluorescence imaging for membrane localization (k), and its quantification (l) of HEK293T cells expressing GSDMD-NT WT or Cys mutants, showing the impairment of C191A in inducing pyroptosis. Scale bars represent 50 μm (g) and 5 μm (l). Data are presented as the mean ± SEM, n = 3 (h-j, l). (c-f) Representative of ≥3 independent experiments. Statistics were measured by two-tailed Student’s t-tests with NS (non-significant) for p > 0.05, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated with 1:1000 anti-FLAG® M2-peroxidase (HRP) antibody, 1:1000 anti-calnexin antibody, and 1:5000 anti-GAPDH antibody for GAPDH loading controls.
Extended Data Fig. 2 GSDMD palmitoylation at Cys191 and its functional effects, and cell death of WT and mutant iBMDMs.
a, UPLC-MS/MS analysis of released ethyl palmitoyl group from Expi293-expressed WT GSDMD (top half, red) and synthetic C16 ethyl palmitate control (bottom half, grey). b, Inputs and cleavage of bacterial expressed and mammalian expressed GSDMD proteins used in liposome leakage assays. All proteins were cleaved equally. c, APE of bacterially expressed recombinant GSDMD, WT and its C191A mutant, detected by the anti-GSDMD antibody CST-39754. Although each PEG-mal used was 10 kDa in size, the molecular weight shift it caused was about twice, as noted previously50. d, Liposome pelleting assay. GSDMD insertion mutant (F184D/L186D) was designed from the GSDMD pore structure8, which should bind to the membrane but does not fully insert into the membrane to form pores. Even at the high concentration used (35 µM) to enable detection by Coomassie staining, the role of palmitoylation in membrane association was apparent. e,f, ABE of undifferentiated THP-1 monocytes (e) or PMA-differentiated THP-1 macrophages (f) treated by LPS, nigericin, or LPS plus nigericin. For differentiated THP-1 cells, nigericin alone and LPS plus nigericin both induced GSDMD cleavage and palmitoylation. g, Palmitoylation by ABE of WT iBMDMs upon treatment by LPS, nigericin, or LPS plus nigericin. h, Western blot of GSDMD in WT THP-1 and in GSDMD KO THP-1 stably reconstituted with GSDMD-GFP by lentiviruses. Expression levels of GSDMD-GFP in reconstituted THP-1 cells were equal to endogenous GSDMD in WT THP-1 cells. i, Western blot of GSDMD in WT iBMDMs and GSDMD KO iBMDM clones stably reconstituted with GSDMD-GFP (WT, C191A, or D275A). Clones of GSDMD-GFP reconstituted iBMDMs with equal expression levels to endogenous GSDMD in WT iBMDMs were selected. The selected clones are labelled in red. j, LDH release of GSDMD KO iBMDMs reconstituted with WT, C191A, or D275A GSDMD-GFP upon treatment by LPS and nigericin. All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics were measured by two-tailed Student’s t-tests with NS (non-significant) for p > 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated with 1:1000 anti-GSDMD antibody.
Extended Data Fig. 3 Regulation of GSDMD palmitoylation and pyroptosis by ROS modulators.
a, GSDMD-NT palmitoylation detected by ABE and quantified in HEK293T cells expressing GSDMD-NT treated or not ROS activators or quenchers. b, GSDMD-NT and calnexin palmitoylation detected by APE in HEK293T cells expressing GSDMD-NT or empty vector. Although the PEG-mal used was 10 kDa in size, the molecular weight shift it caused is roughly twice, as noted previously50. c, d, LDH release (c), and cell viability (d) of HEK293T cells expressing GSDMD-NT and treated or not with ROS modulators. e, Anti-FLAG immunofluorescence imaging of HEK293T cells expressing GSDMD-NT and treated with ROS modulators, showing increased cell membrane localization by Rot and AMA and its inhibition by 2-BP, as well as the diffuse cytoplasmic localization upon treatment by NAC. Nuclei are marked by the DNA dye DAPI. Scale bars represent 5 μm. f, Plot of PI positivity against % GSDMD palmitoylation, showing an approximately linear relationship. g, Measurement of cellular oxidative stress by CellROX in THP-1 cells treated or not with LPS plus nigericin, and with ROS and palmitoylation modulators. h-l, PI staining (h), LDH release (i), cell viability (j), IL-1β secretion (k), and membrane staining (l) of THP-1 cells treated or not with LPS + nigericin and with ROS modulators. Scale bar represent 50 μm (h). All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics were measured by two-tailed Student’s t-tests with * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Scale bars represent 5 μm (e) and 50 μm (h). Immunoblots were incubated with 1:1000 anti-FLAG® M2-peroxidase (HRP) antibody, 1:1000 anti-calnexin antibody, and 1:5000 anti-GAPDH antibody for GAPDH loading controls.
Extended Data Fig. 4 GSDMD targets the mitochondria to induce ROS.
a, Pulldown of proteins with oxidized Cys residues in LPS primed and nigericin activated THP-1 cells followed by anti-GSDMD western blot (top). GSDMD is minorly oxidized in primed and activated cells even with additional ROS activators (≤5%). The pulldown and the input lanes were run on the same gel (bottom). b,c, Imaging (b) and quantification (c) of cellular oxidative stress by CellROX and MitoSOX in GSDMD KO THP-1 cells electroporated with GSDMD-NT WT or C191A. WT GSDMD-NT expression leads to higher cellular oxidative stress than C191A mutant. Data are presented as the mean ± SEM, n = 3. Scale bars represent 80 μm (b). d,e, Measurement of cellular and mitochondrial stress by CellROX (d), and MitoSOX (e) intensity in GSDMD KO THP-1 cells reconstituted with WT, C191A, or D275A (cleavage deficient) GSDMD upon treatment by DMSO, LPS or LPS and nigericin. C191A GSDMD-reconstituted cells did not enhance ROS production upon stimulation by LPS and nigericin as compared to WT GSDMD, while D275A GSDMD-reconstituted cells showed significantly enhanced ROS production. Expression levels of GSDMD-GFP in reconstituted THP-1 cells were equal to endogenous GSDMD in WT THP-1 cells. Data are presented as the mean ± SEM, n = 3. f,g, Measurement of cellular oxidative stress by CellROX (f), and MitoSOX (g) of GSDMD KO THP-1 cells treated with LPS, LPS and nigericin, or with additional ROS activators or quenchers, highlighting the GSDMD dependence of ROS generation during inflammasome activation. Data are presented as the mean ± SEM, n = 3. h,i, Palmitoylated GSDMD detected by ABE in isolated mitochondria from THP-1 cells (h) and iBMDMs (i) before and after inflammasome activation. j, Western blots of GSDMD in isolated mitochondria from WT iBMDMs, Crls1 KO iBMDMs, or Plscr3 KO iBMDMs, showing lack of GSDMD in mitochondria from the KO cells. k,l, ROS measured by MitoSox (k) or CellRox (l) showing close to baseline ROS in the KO iBMDMs even upon inflammasome activation, in contrast to WT cells. (h-j) Representative of ≥3 independent experiments. Data are presented as the mean ± SEM, n = 3. Statistics were measured by two-tailed Student’s t-tests with NS (non-significant) for p > 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. All immunoblots were incubated with 1:1000 anti-GSDMD antibody, and 1:1000 anti-COX IV antibody for COX IV loading controls.
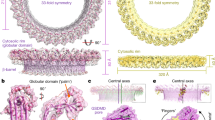
Extended Data Fig. 5 Cryo-EM structure of palmitoylated, full-length GSDMD.
a,b, Negative staining EM images of GSDMD pores by D275A GSDMD-FL (a), or empty liposomes (b) on liposomes (left) and after detergent solubilization (right). Red arrowheads indicate a few examples of pores. c, Cryo-EM workflow of GSDMD-FL pore structure. d, FSC curve of the cryo-EM reconstruction. e, Overall view of the cryo-EM density segmented to show GSDMD-NT in cyan. f, The globular rim of the GSDMD-NT structure is higher, shown by the ribbon diagram extending above the cryo-EM density of the GSDMD-NT region of the GSDMD-FL pore. g, The globular rim domain assembly in the pre-pore structure of GSDMD-NT (top) and its fitting into the density of full-length GSDMD pore. h, FSC curve for the cryo-EM structure of palmitoylated GSDMD. The resolution is likely over-estimated as we cannot visualize individual helices in the map. i, j, Cryo-EM map of palmitoylated GSDMD (grey) fitted separately with NT (cyan) and CT (green) from unpalmitoylated GSDMD structure (PDB: 6N9O) (i), and the comparison of the palmitoylated model with the unpalmitoylated GSDMD structure (j). The NT-CT interaction is less compact in the palmitoylated GSDMD structure, suggesting partial overcome of autoinhibition.
Extended Data Fig. 6 GSDMD KO THP-1 cells with uncleavable D275A GSDMD-FL induced ROS, pyroptosis, and membrane localization, and a GSDMD disease mutant enhanced cell death in a palmitoylation-dependent manner.
a,b, ROS activators alone induced significant LDH release (a) and reduced cell viability (b) in GSDMD KO THP-1 stably reconstituted with GSDMD-GFP D275A or WT GSDMD. Expression levels of GSDMD-GFP in reconstituted THP-1 cells were equal to endogenous GSDMD in WT THP-1 cells. c,d, Palmitoylation in THP-1 cells treated or not with ROS activators or quencher (c), or primed and activated and treated or not with ROS activators or quencher (d). e-j, ROS measured by MitoSox (e), palmitoylation (f), LDH release (g), cell viability (h), IL-1β release (i), and cellular ROS (j) in GSDMD KO THP-1 cells or GSDMD KO THP-1 cells reconstituted with D275A, treated or not with ROS activators or quenchers. Expression levels of GSDMD-GFP in reconstituted THP-1 cells were equal to endogenous GSDMD in WT THP-1 cells. All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics used two-tailed Student’s t-tests, with NS (non-significant) for p > 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated 1:5000 anti-GAPDH antibody for GAPDH, and 1:1000 anti-GSDMD antibody.
Extended Data Fig. 7 GSDMD KO iBMDMs reconstituted with uncleavable D275A GSDMD-FL induce ROS, pyroptosis, and membrane localization upon inflammasome activation and/or ROS activators.
a, Palmitoylation in iBMDMs treated or not with ROS activators or quencher. b,c, ROS activators alone induced significant PI positivity as measured by Incucyte® (b) and increased LDH release (c) in GSDMD KO iBMDMs stably reconstituted or not with GSDMD-GFP D275A or WT GSDMD. d-f, Palmitoylation (d), PI positivity (e), and MitoSOX (f) in GSDMD KO iBMDMs reconstituted with GSDMD-GFP D275A, clone 5, activated by LPS and nigericin, and treated or not with additional ROS activators, quenchers, or 2-BP. g,h, GFP imaging (g) and quantification (h) for GSDMD membrane localization in clone 5 of GSDMD KO iBMDMs reconstituted with GSDMD-GFP D275A upon inflammasome activation and with or not ROS modulators and 2-BP. Scale bars represent 5 μm (g). All results were obtained from at least 3 independent experiments. GSDMD KO iBMDM clones reconstituted with D275A that had equal expression levels to endogenous GSDMD in WT iBMDMs were selected. Error bars represent SEM. Statistics used two-tailed Student’s t-tests, with NS (non-significant) for p > 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated with 1:1000 anti-GSDMD antibody and 1:5000 anti-GAPDH antibody for GAPDH loading controls.
Extended Data Fig. 8 Primary BMDMs induce increased palmitoylation and pyroptosis upon inflammasome activation and/or ROS activators.
a-d, Palmitoylation (a,c) and PI positivity (b,d) in primary WT and Gsdmd KO BMDMs (a,b), or primary caspase-1/11 dKO and Gsdmd KO BMDMs (c,d) treated or not with 2-BP, ROS activators or quenchers. e, GSDMD palmitoylation by ROS activators or 2-BP in primary WT and caspase-1/11 dKO BMDMs. f, ROS activators alone increased LDH release in both WT and caspase-1/11 dKO primary BMDMs, but not in Gsdmd KO BMDMs. g-i, Time course as measured by Incucyte® of PI (g,i), or Annexin V positivity (h) with ROS induction without LPS and nigericin treatment in primary WT, Gsdmd KO, and caspase-1/11 dKO BMDMs. All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics used two-tailed Student’s t-tests, or two-way ANOVA (g-i) with NS (non-significant) for p > 0.05, * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated with 1:1000 anti-GSDMD antibody and 1:5000 anti-GAPDH antibody for GAPDH loading controls.
Extended Data Fig. 9 GSDMD palmitoylation and cell death in primary BMDMs.
a, GSDMD palmitoylation detected by ABE in primary caspase-1/11 dKO BMDMs upon dsDNA electroporation, flatox treatment or LPS electroporation, and treated or not with ROS activator Rot, 2-BP, or ROS quencher NAC. b-g, PI positivity (b,d,f) and LDH release (c,e,g) in primary caspase-1/11 dKO BMDMs and primary GSDMD KO BMDMs upon AIM2 inflammasome activation by dsDNA electroporation (b,c), NAIP5/NLRC4 inflammasome activation by flatox treatment (d,e), and non-canonical inflammasome activation by LPS electroporation (f,g). All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics were measured by two-tailed Student’s t-tests with * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
Extended Data Fig. 10 Identification of ZDHHC5 and ZDHHC9 as the main palmitoyltransferases for GSDMD palmitoylation and their regulation.
a, Palmitoylation as measured by ABE of GSDMD-NT expressed in HEK293T cells cotransfected with pooled siRNAs for ZDHHC5 and ZDHHC9, or all human ZDHHCs except ZDHHC5/9. b, Co-localization imaging analysis of ZDHHC5-YFP and ZDHHC9-YFP with GSDMD-mCherry upon co-expression in HEK293T cells. Hoechst (blue) stained nuclei. The cap-like structures appear to be intact Golgi. Scale bars represent 5 μm. c, Anti-FLAG pulldown controls. ZDHHS5 and ZDHHS9 were not pulled down without co-expression with the GSDMD-FLAG bait, and GSDMD-FLAG did not pull down ZDHHS5 and ZDHHS9 without the expression of ZDHHS5 and ZDHHS9. d,e, Cell viability (d), and PI positivity (e) of GSDMD-NT expressing HEK293T cells upon siRNA knockdowns of ZDHHCs. Other pooled siRNA contained siRNAs for all human ZDHHCs except ZDHHC5/9. f, Anti-FLAG immunofluorescence imaging of HEK293T cells with siRNA knockdowns of ZDHHC5, ZDHHC9, or both expressing GSDMD-NT-FLAG. Only cells treated with scRNA showed strong cell surface staining. Nuclei are marked by the DNA dye DAPI. Scale bars represent 5 μm. g, Quantification of GSDMD palmitoylation in Fig. 5f detected by ABE in THP-1 cells upon siRNA knockdown of ZDHHC5, ZDHHC9, or both and treatment with LPS plus nigericin, showing that these knockdowns compromised GSDMD palmitoylation. h-j, LDH release (h), cell viability (i), and IL-1β release (j) of THP-1 cells upon siRNA knockdown of ZDHHC5, ZDHHC9, both, or other ZDHHCs and treatment with LPS plus nigericin. k-m, Palmitoylation as measured by ABE of GSDMD (k), PI positivity (l) and LDH release (m) of WT THP-1 cells in the presence of pooled siRNAs for ZDHHC5 and ZDHHC9, or all human ZDHHCs except ZDHHC5/9. n, Western blots of THP-1 cells with single and double knockouts of ZDHHC5 or ZDHHC9, or both. o,p, Palmitoylation (o), and LDH release (p) of ZDHHC knockout THP-1 cell clones primed and activated with LPS and nigericin. All results were obtained from at least 3 independent experiments. Error bars represent SEM. Statistics were measured by two-tailed Student’s t-tests with NS (non-significant) for p > 0.05, *** for p < 0.001, and **** for p < 0.0001. Immunoblots were incubated 1:1000 anti-FLAG® M2-peroxidase (HRP) antibody (a, c), 1:5000 anti-GAPDH antibody for GAPDH (a, k, o), 1:1000 anti-ZDHHC5 antibody (c), 1:1000 anti-ZDHHC9 antibody (c), 1:500 anti-ZDHHC5 antibody (n), 1:500 anti-ZDHHC9 antibody (n), 1:1000 anti-β-actin antibody (n), and 1:1000 anti-GSDMD antibody (k, o).
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1–12, Supplementary Tables 1 and 2 and the legends for Supplementary Videos 1 and 2.
Supplementary Video 1
Videos of confocal stacks of GSDMD-KO THP-1 cells reconstituted with endogenous level of GSDMD–GFP (green) and stained with PI (red) after LPS/nigericin and DMSO treatment.
Supplementary Video 2
Videos of confocal stacks of GSDMD-KO THP-1 cells reconstituted with endogenous level of GSDMD–GFP (green) and stained with PI (red) after LPS/nigericin and 2-BP treatment.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, G., Healy, L.B., David, L. et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature (2024). https://doi.org/10.1038/s41586-024-07373-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07373-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.