Abstract
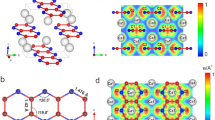
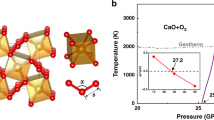
Under standard conditions, carbon dioxide (CO2) is a simple molecular gas and an important atmospheric constituent, whereas silicon dioxide (SiO2) is a covalent solid, and one of the fundamental minerals of the planet. The remarkable dissimilarity between these two group IV oxides is diminished at higher pressures and temperatures as CO2 transforms to a series of solid phases, from simple molecular to a fully covalent extended-solid V, structurally analogous to SiO2 tridymite. Here, we present the discovery of an extended-solid phase of CO2: a six-fold coordinated stishovite-like phase VI, obtained by isothermal compression of associated CO2-II (refs 1,2) above 50 GPa at 530–650 K. Together with the previously reported CO2-V (refs 3–5) and a-carbonia6, this extended phase indicates a fundamental similarity between CO2 (a prototypical molecular solid) and SiO2 (one of Earth’s fundamental building blocks). We present a phase diagram with a limited stability domain for molecular CO2-I, and suggest that the conversion to extended-network solids above 40–50 GPa occurs via intermediate phases II (refs 1,2), III (refs 7,8) and IV (refs 9,10). The crystal structure of phase VI suggests strong disorder along the c axis in stishovite-like P42/m n m, with carbon atoms manifesting an average six-fold coordination within the framework of s p3 hybridization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Iota, V. & Yoo, C. S. Phase diagram of carbon dioxide: Evidence for a new associated phase. Phys. Rev. Lett. 86, 5922–5925 (2001).
Yoo, C. S. et al. Crystal structure of pseudo-six-fold carbon dioxide phase II at high pressures and temperatures. Phys. Rev. B 65, 104103 (2002).
Iota, V., Yoo, C. S. & Cynn, H. Quartzlike carbon dioxide: An optically nonlinear extended solid at high pressures and temperatures. Science 283, 1510–1513 (1999).
Sera, S., Corazon, C., Chiarotti, G. L., Scandolo, S. & Tossatti, E. Pressure-induced solid carbonates from molecular CO2 by computer simulation. Science 284, 788–790 (1999).
Yoo, C. S. et al. Crystal structure of carbon dioxide at high pressure: “Superhard” polymeric carbon dioxide. Phys. Rev. Lett. 83, 5527–5530 (1999).
Santoro, M. et al. Amorphous silica-like carbon dioxide. Nature 441, 857–860 (2006).
Aoki, K., Yamawaki, H., Sakashita, M., Gotoh, Y. & Takemura, K. Crystal structure of the high-pressure phase of solid CO2 . Science 263, 356–358 (1994).
Olijnyk, H. & Jephcoat, A. P. Vibrational studies on CO2 up to 40 GPa by Raman spectroscopy at room temperature. Phys. Rev. B 57, 879–888 (1998).
Yoo, C. S., Iota, V. & Cynn, H. Nonlinear carbon dioxide at high pressures and temperatures. Phys. Rev. Lett 86, 444–447 (2001).
Park, J.-H. et al. Crystal structure of bent carbon dioxide phase IV. Phys. Rev. B 68, 014107 (2003).
Gorelli, F. A., Giordano, V. M., Salvi, P. R. & Bini, R. Linear carbon dioxide in the high-pressure high-temperature crystalline phase IV. Phys. Rev. Lett. 93, 205503 (2004).
Kuchta, B. & Etters, R. Generalized free-energy method used to calculate the high-pressure, high-temperature phase transition in solid CO2 . Phys. Rev. B 47, 14691–14695 (1993).
Sinclair, W. & Ringwood, A. E. Single crystal analysis of the structure of stishovite. Nature 272, 714–715 (1978).
Dong, J. et al. Investigation of hardness in tetrahedrally bonded nonmolecular CO2 solids by density-functional theory. Phys. Rev. B 62, 14685–14689 (2000).
Holm, B., Ahuja, R., Belonoshko, A. & Johansson, B. Theoretical investigation of high pressure phases of carbon dioxide. Phys. Rev. Lett. 85, 1258–1261 (2000).
Hemley, R. J., Mao, H. K. & Chao, E. C. T. Raman spectrum of natural and synthetic stishovite. Phys. Chem. Minerals 13, 285–290 (1986).
Mammone, J. F., Nicol, M. & Sharma, S. K. Raman spectra of TiO2-II, TiO2-III, SnO2, and GeO2 at high pressure. J. Phys. Chem. Solids 42, 379 (1981).
Yoo, C. S. in Science and Technology of High Pressure Vol. 1 (eds Manghnani, M. H., Nellis, W. J. & Nicol, M. F.) 86–89 (Univ. Press, Hyderabad, India, 2000).
Tassone, F., Chiarotti, G. L., Rousseau, R., Scandolo, S. & Tosatti, E. Dimerization of CO2 at high pressure and temperature. ChemPhysChem 6, 1752–1756 (2005).
Cohen, R. E. in High Pressure Research: Application to Earth and Planetary Sciences (eds Syono, Y. & Manghnani, M. H.) 425 (Terra Scientific, AGU, Washington DC, 1992).
Santillan, J. & Williams, Q. A high-pressure infrared and X-ray study of FeCO3 and MnCO3: comparison with CaMg(CO3)2-dolomite. Phys. Earth Planet. Inter. 143/144, 291–304 (2004).
Harvey, R. P. & Mcsween, H. Y. A possible high-temperature origin for the carbonates in the martian meteorite ALH84001. Nature 382, 49–51 (1996).
Acknowledgements
The work has been supported by the LDRD and PDRP programs at the LLNL, University of California, under the auspices of the US-DOE under contract number W-7405-ENG-48. The X-ray work was done by using the High Pressure Collaborating Access Team’s micro-diffraction beamline (16IDB) of the Advanced Photon Source. Use of the HPCAT facility was supported by DOE-BES, DOE-NNSA (CDAC, LLNL, UNLV), NSF, DOD-TACOM and the W.M. Keck Foundation.
Author information
Authors and Affiliations
Contributions
Project planning: V.I., C.S.Y., samples and Raman measurements: V.I., Z.J., XRD measurements: V.I., C.S.Y., W.E., H.C., data analysis: V.I., C.S.Y., J.H.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure 1 (PDF 85 kb)
Rights and permissions
About this article
Cite this article
Iota, V., Yoo, CS., Klepeis, JH. et al. Six-fold coordinated carbon dioxide VI. Nature Mater 6, 34–38 (2007). https://doi.org/10.1038/nmat1800
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1800
This article is cited by
-
Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestion for the formation of amorphous nitrogen
Nature Communications (2023)
-
Thermal Pressure in the Thermal Equation of State for Solid and a Proposed Substitute
International Journal of Thermophysics (2022)
-
Predicting the phase diagram of solid carbon dioxide at high pressure from first principles
npj Quantum Materials (2019)
-
Pressure-Induced Crystallization and Phase Transformation of Para-xylene
Scientific Reports (2017)
-
Diamond formation in the deep lower mantle: a high-pressure reaction of MgCO3 and SiO2
Scientific Reports (2017)