Abstract
In this study, we sought to evaluate the influence of positive pathogens in stool (PPS) on clinical outcomes in critical ill patients with Sepsis-associated acute kidney injury (S-AKI) from intensive care unit. Our sample consisted of 7338 patients, of whom 752 (10.25%) had PPS. We found that the presence of Clostridium difficile (C. difficile) and protists in stool samples was correlated with survival during hospitalization, as well as 30-day and 90-day survival. Interestingly, there was no significant difference in overall survival and 30-day in-hospital survival between the PPS group and the negative pathogens in stool (NPS) control group. However, the cumulative incidence of 90-day infection-related mortality was significantly higher in the PPS group (53 vs. 48%, P = 0.022), particularly in patients with C. difficile in their stool specimens. After adjusting for propensity scores, the results also have statistical significance. These findings suggest that PPS may affect the 90-days survival outcomes of S-AKI, particularly in patients with C. difficile and protists in their stool samples. Further research is warranted to further explore these associations.
Similar content being viewed by others
Introduction
Sepsis association acute kidney injury (AKI) is a multifactorial clinical syndrome and a serious complication in critically ill patients1,2,3. It is associated with high mortality and increased healthcare costs. The mortality rate among AKI patients receiving renal replacement therapy (RRT) exceeds 50%, and the pathogenesis of this condition is not fully understood4,5. Sepsis is the leading cause of AKI in the intensive care unit (ICU). It can result from the destruction of the patient's mucosal barrier, an imbalance in the intestinal flora, the use of antibiotics and a lack of intestinal nutrition6,7. These factors can induce inflammatory responses that compromise host metabolism and immunity and promote the development of AKI. Several studies have demonstrated an association between gut microbiota and kidney injury, suggesting a close link between the kidneys and the gastrointestinal tract, known as the gut-kidney axis8,9,10. Dysregulated interactions between the intestinal epithelium, immune system, endogenous pathogens and kidneys may exacerbate systemic inflammation and AKI. Therefore, the relationship between stool pathogens and sepsis association AKI is of great importance. It can guide the subsequent treatment of patients with kidney disease and help reverse gastrointestinal dysfunction.
The gut-kidney axis has received considerable attention in recent years due to the intriguing interactions between these two organ systems. Acute kidney damage can be caused by pathogens in the intestines11. Several studies have investigated the impact of the stool pathogens on the progression of sepsis association AKI to chronic kidney disease (CKD)12,13,14,15. Given that most patients with sepsis are routinely treated with antibiotics, this could potentially lead to an imbalance in the intestinal flora and result in dynamic changes in the bacterial flora. A positive pathogen in stool (PPS) may reflect the dominant microorganisms in the gastrointestinal tract. The stool pathogens of patients with treated sepsis association AKI may be altered during transplantation due to intensive conditioning and administration of therapeutic antibiotics16.
However, the role of stool pathogens in sepsis-associated AKI has not been established by studies. The aim of this retrospective study was to determine the impact of stool pathogens on clinical outcomes in patients with sepsis-associated AKI. To control for confounders, we also performed propensity score matching (PSM) and causal inference for the analysis.
Results
Results of stool cultures
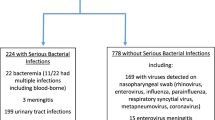
A total of 27,234 stool samples were collected from 7338 patients. A PPS was observed in 752 patients (10.25%), whose age, weight, and sex were comparable to those of the persistently negative pathogens in stool (NPS) group (Table 1). The most common pathogen identified in stool samples was C. difficile, followed by Adenovirus, Enterococcus sp., and Salmonella species (Table 2). One patient’s staphylococcal sample contained Methicillin-resistant Staphylococcus. The most common virus identified from stool samples was Adenovirus, while the most common Enterobacteriaceae was Salmonella. Multiple pathogens (≥ 2) were detected in 4 patients.
The impact of PPS on sepsis AKI
There was no statistical difference between the SOFA and GCS scores, indicating a similar level of critical illness in both groups of patients (Table 1, P > 0.05). The median length of hospital stays for the NPS and PPS groups was 17 days (range: 10–29) and 22 days (range: 12–35), respectively (Table 1, P < 0.001). In-hospital mortality was calculated using the chi-squared test and showed a statistically significant difference (Table 1, P = 0.04). The mortality rate for patients with a positive stool test was 28% compared to 25% for patients with a negative stool test. There was no statistically significant difference observed between the white blood cells (WBC) and platelets (Table 1, P > 0.05). There was also no statistical difference in the number of using RRT treatment between the two groups (Table 1, P > 0.05). Regarding the sources of infectious pathogens, there are certain statistical differences between gram-negative bacteria and gram-positive bacteria. There were statistical differences in the source of infection, but most of the infections in the two groups occurred in the respiratory and blood systems (Table1, P < 0.05). There was a statistically significant difference in the time it took to test for fecal pathogens between the two groups, with the PPS group taking an average of 6 days and the NPS group taking an average of 8 days (Table1, P < 0.001).
Impact of PPS on 90-Day survival, hospital survival, and 30-day survival
The KM curves demonstrate that mortality in patients with sepsis-associated AKI was similar between the NPS (48.45%) and PPS (52.93%) groups (P = 0.01; Fig. 1A). The 30-day cumulative mortality rate in the PPS group was 30.85%, which was higher than the 28.35% in the NPS group (P = 0.16; Fig. 1B). The probability of death during hospitalization in the PPS group (42.25%) was slightly higher than in the NPS group (43.69%; P = 0.21; Fig. 1C). The KM curves after PSM of hospital survival, 30-day survival, and 90-day survival are presented in Fig. 1D–F. Differences between the two groups after PSM did not alter our results. Enterobacteriaceae and Enterococci in stool samples were not associated with patient survival during hospitalization or with 30-day and 90-day survival. However, the presence of C. difficile in stool samples was significantly associated with decreased 90 days survival (Fig. 2A,B, Table 3). We performed causal inference of C. difficile positivity and 90-day prognosis, removed confounders, and drew a DAG diagram (Supplementary Fig. 1). After PSM analysis, the average treatment effect was 0.05 (95% CI 0.01–0.08, P < 0.05). This means that patients who develop C. difficile stool positivity have a 5% lower 90-day survival rate than those whose stool without C. difficile.
Variables that showed differences in 90-day patient survival were analyzed using single-factor Cox analysis with stool culture. Supplementary Table 1 demonstrates all variables initially considered in the forward stepwise model. The correlation between these factors and survival was statistically significant (Table 4).
Because treatment is already in using at the time of stool examination, antibiotics have an effect on fecal pathogens. The use of antibiotics was described in both groups (Table 5). In the group with positive pathogenic bacteria, vancomycin was used more frequently, with about 88% of patients using it, and there was a statistical difference between the two groups. Similarly, metronidazole was used more often in the PPS group and less often in the NPS group (P < 0.001). Penicillin/cephalosporin and quinolones were used less in the PPS group than in the NPS group (P < 0.05). There was no statistical significance in the antibiotic use of macrolides, sulfonamides, tetracyclines and aminoglycosides between the two groups (P > 0.05).
Discussion
In this study, we observed that the presence of C. difficile protists in stool samples was associated with a higher risk of in-hospital death, 30-day death, and 90-day death in patients with sepsis-associated AKI. PPS is associated with poor survival, particularly in those with C. difficile in their stool samples. To our knowledge, this study provides the first opportunity to investigate the exploratory role of PPS in patients with sepsis-associated AKI.
In this study, we observed that the presence of C. difficile and protists in stool samples significantly increased the risk of 90-day mortality. Protists were associated with worse in-hospital and 30-day survival. Multiple stool tests are necessary to avoid false negatives, so we did not include confounders in the number of tests17. Taking into account prolonged hospitalization with multiple fecal examinations, a worse 90-day survival was observed in PPS patients after propensity score matching to adjust for the possible influence of confounders.
C. difficile and protists in stool samples is associated with sepsis association AKI, and infections, and dysbiosis may negatively impact their survival18,19. This phenomenon also is due to increased levels of inflammatory cytokines and the fact that ischemia can lead to changes in the constituent organisms that make up the intestinal pathogens20,21. Sepsis can lead to a significant loss of microbial diversity in patients22. In our study, patients with elevated absolute neutrophil counts had lower survival rates than patients with decreased absolute neutrophil counts. Gastrointestinal colonization with C. difficile may be a potential risk factor for invasive fungal in sepsis-associated AKI patients. However, common dysbiosis pathogenic bacterial infections were not found in this study, like candidiasis. Therefore, patients with sepsis-associated AKI should be monitored for the presence of C. difficile in stool samples.
Patients whose stool samples contained C. difficile had worse survival outcomes than those with persistently NPS. In patients with sepsis-associated AKI, those whose intestines are colonized with antibiotic-resistant bacteria are likely to develop bacteremia. Inhibiting the intestinal flora can significantly reduce the incidence of bacteremia. High concentrations of C. difficile bacteria in feces can result in a high risk of bacterial translocation into the bloodstream. Microbial abundance and diversity in hemodialysis patients are on a downward trend. In addition, the mortality rate of sepsis-associated AKI patients undergoing further kidney replacement therapy is high, reaching up to 50%23. Broad-spectrum antibiotics increase the risk of Clostridium difficile infection. Therefore, reducing the use of high-risk antibacterial drugs associated with Clostridium difficile infection (such as second- and third-generation broad-spectrum cephalosporins, fluoroquinolones, clindamycin, etc.), as well as their frequency and duration, may improve patient clinical outcomes24,25.
Protists organisms constitute a heterogeneous category of eukaryotic microbes that bear a potential association with heightened mortality in individuals suffering from sepsis-related acute kidney injury26. The poor prognosis may be due to the severe immune stress caused by protists27. For instance, protozoan infections can lead to endothelial dysfunction, hinder perturb the integrity of the glycocalyx layer as well as foster platelet aggregation and the initiation of coagulation cascades culminating in fibrin deposition28. Our results resonate with prior studies, affirming the contributory role of protists in augmenting patient fatality rates.
In the ICU, patients with sepsis association AKI have lower long-term 90-day survival. Our analysis showed that patients who were positive pathogens in stool had lower 90-day survival rates. This is somewhat related to treatment. Vancomycin, a potent antibiotic that primarily targets Gram-positive bacteria, is particularly effective against methicillin-resistant Staphylococcus aureus (MRSA) and other drug-resistant staphylococci29. In the ICU, where patients with sepsis have PPS, the increased use of vancomycin may be indicative of efforts to control C. difficile infections rather than association with sepsis-associated acute kidney injury (AKI). In addition, our multivariate regression showed that it was associated with 90-day survival and also associated with quinolones. Quinolones are used less frequently in patients with positive stools, which may indicate the need for caution in clinical use. The source of the fungal infection also has a negative impact on the patient's prognosis for 90-day survival. Due to the patient's compromised immune function, it is difficult for the host to eliminate fungi, toxins, and metabolites30,31. The antibiotics used have a certain nephrotoxicity, resulting in a worse 90-day prognosis than in normal patients.
In the context of clinical implications, the detection of C. difficile and protists in stool samples, which are associated with adverse survival outcomes, indicates a recommendation for routine fecal microbiota screening in these patients, particularly during the early phase of hospitalization. Enhanced testing frequency minimizes false-negative results, enabling prompt identification and intervention in cases of potential microbial imbalance. In light of the observed association between antibiotic use and PPS, clinicians may wish to exercise caution when prescribing second- and third- generation cephalosporins, fluoroquinolones, clindamycin, and other high-risk antibiotics. It may be advisable to consider shorter courses where feasible. Treatment strategies should prioritize alternatives with reduced nephrotoxicity to safeguard both renal function and the integrity of the gut microbiome. Maintaining microbial diversity and gut microbiome stability is vital to prevent bacterial translocation and secondary infections. Moreover, it is imperative to conduct large-scale, prospective, multi-center studies to validate these observations and elucidate the correlations between gut microbiota and clinical outcomes across diverse populations. These endeavors will facilitate the development of more precise therapeutic guidelines, tailored to the unique microbial profiles of patients with sepsis-associated acute kidney injury.
A limitation of this study is its retrospective design coupled with a relatively small sample of bacterial species in the PPS group. Therefore, the role of microorganisms in fecal samples from patients with sepsis-associated AKI warrants further investigation. Second, this study was conducted with patients in the United States, where bacterial prevalence may vary. This variability could potentially influence the association between fecal microorganisms and clinical outcomes in other populations. Third, retrospective studies may have inherent flaws. For example, selection bias, included patients mainly collected stool after treatment, and treatment caused different causes of stool. Prospective studies are needed to investigate the correlation between the presence of stool pathogens at admission and prognosis.
In conclusion, our results suggest that PPS significantly influence the 90 days survival outcomes of patients with sepsis association AKI, particularly those with C. difficile in their stool samples. Depending on the differential detection of positive and negative antibiotics in stool samples, clinicians may find it beneficial to adjust their antimicrobial therapy regimens accordingly.
Methods
Patients
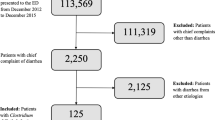
This study conducted a retrospective analysis of the MIMIC database, a comprehensive resource for intensive care patients32. The MIMIC database was established in 2003 through a collaborative effort involving the Beth Israel Deaconess Medical Center (BIDMC), MIT, the National Institutes of Health, Massachusetts General Hospital, emergency physicians, critical care physicians, computer scientists, and other critical care professionals. The current version, MIMIC-IV, includes patients admitted between 2008 and 2019. Prior to utilizing the database, our team completed the necessary training and obtained the appropriate certifications. As this project does not impact clinical care, all sensitive health information remains anonymous, thereby eliminating the need for individual patient consent. We followed the official MIMIC-IV tutorial to construct the research database using PostgreSQL (version 10.0, PostgreSQL Global Development Group). We extracted data from patients with sepsis-associated AKI who underwent comprehensive stool culture analysis for all bacteria and fungi in the gastrointestinal tract. The exclusion criteria for this study are as follows:1) Patients who are younger than 18 years old; 2) Patients with missing AKI diagnostic information, creatinine value, or urine volume. 3) There is a lack of information regarding the diagnosis of sepsis, and there is no record of the SOFA score or infection information. 4) There are no records of stool or digital rectal examinations for the patients. A total of 7338 patients were included (Table 1). Figure 3a shows the patient screening flowchart, while Fig. 3b illustrates various stool pathogen tests.
Definitions and assessments
Patients with sepsis were selected and diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)33. In addition, we compiled additional codes as recommended by MIMIC-IV. Acute kidney injury (AKI) was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria, which specify that AKI is either an increase of 0.3 mg/dl within 48 h or a 50% increase over baseline serum creatinine (sCr)34. The primary endpoint was 90-day survival. Secondary endpoints included in-hospital death and 30-day survival. The definition of positive pathogens in stool, during this ICU treatment, positive pathogens found in stool, digital rectal examination, and fecal swab were defined as positive pathogens in stool.
Statistical analysis
Missing values were imputed multiple times using the 'mice' package (version 3.15.0) in R. Baseline variables were compared using independent samples t-tests or Mann–Whitney U tests as appropriate. Survival probabilities were estimated using the Kaplan–Meier (KM) method. Potential prognostic factors were assessed by multivariate analysis using Cox proportional hazards regression and stepwise model selection methods. To illustrate the relationship between these three variables, we used a Directed Acyclic Graph (DAG). Because there were multiple stool tests for a long hospital stay and only one test for a short hospital stay, we considered length of treatment in hospital as a confounding factor and performed propensity score matching. Propensity scores were then used to control for confounding by R package ‘MatchIt’. The average treatment effect (ATE) was calculated using inverse probability weighting in this study. The multivariate Cox proportional hazards model included variables with statistically significant differences in individual factors. The ATE represents the causal effect of the variable of interest on patient 90 days causal inference radio in both the death and non-death study populations.
Independent variables with P > 0.05 were sequentially excluded from the model, while independent variables with P < 0.05 were considered statistically significant. The 'CBCgrp' package (version 2.8.2) in R (version 4.1.2) was used to calculate data35. All reported P-values are based on two-tailed hypothesis testing. Data analysis was primarily performed using competing risk analysis in the R package (version 4.1.2).
Data availability
The data is open source and freely available at https://www.physionet.org/content/mimiciv/2.2.
References
Mandelbaum, T. et al. Outcome of critically ill patients with acute kidney injury using the acute kidney injury network criteria*. Crit. Care Med. 39, 2659 (2011).
Kaddourah, A., Basu, R. K., Bagshaw, S. M. & Goldstein, S. L. Epidemiology of acute kidney injury in critically Ill children and young adults. N. Engl. J. Med. 376, 11–20. https://doi.org/10.1056/NEJMoa1611391 (2017).
Vijayan, A. et al. Recovery after critical illness and acute kidney injury. Clin. J. Am. Soc. Nephrol. 16, 1601–1609. https://doi.org/10.2215/cjn.19601220 (2021).
Fuhrman, D. Y., Kane-Gill, S., Goldstein, S. L., Priyanka, P. & Kellum, J. A. Acute kidney injury epidemiology, risk factors, and outcomes in critically ill patients 16–25 years of age treated in an adult intensive care unit. Ann. Intensive Care 8, 26. https://doi.org/10.1186/s13613-018-0373-y (2018).
Barbar, S. D. et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N. Engl. J. Med. 379, 1431–1442. https://doi.org/10.1056/NEJMoa1803213 (2018).
Zhang, J. et al. Gut-kidney crosstalk in septic acute kidney injury. Crit. Care 22, 117. https://doi.org/10.1186/s13054-018-2040-y (2018).
Sun, T., Wang, L. & Zhang, H. Intestinal microbiota in sepsis. Intensive Care Res. 2, 1–7. https://doi.org/10.1007/s44231-022-00001-8 (2022).
Saranya, G. R. & Viswanathan, P. Gut microbiota dysbiosis in AKI to CKD transition. Biomed. Pharmacother. 161, 114447. https://doi.org/10.1016/j.biopha.2023.114447 (2023).
Kobayashi, T., Iwata, Y., Nakade, Y. & Wada, T. Significance of the gut microbiota in acute kidney injury. Toxins https://doi.org/10.3390/toxins13060369 (2021).
Lei, J., Xie, Y., Sheng, J. & Song, J. Intestinal microbiota dysbiosis in acute kidney injury: novel insights into mechanisms and promising therapeutic strategies. Ren. Fail. 44, 571–580. https://doi.org/10.1080/0886022X.2022.2056054 (2022).
Saithong, S. et al. Candida administration worsens neutrophil extracellular traps in renal ischemia reperfusion Injury mice: An impact of gut fungi on acute kidney injury. J. Innate Immun. 14, 502–517. https://doi.org/10.1159/000521633 (2022).
Bäumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. https://doi.org/10.1038/nature18849 (2016).
Rysz, J. et al. The impact of CKD on uremic toxins and gut microbiota. Toxins 13, 252 (2021).
Hauser, A. B., Stinghen, A. E. M., Gonçalves, S. M., Bucharles, S. & Pecoits-Filho, R. A gut feeling on endotoxemia: Causes and consequences in chronic kidney disease. Nephron. Clin. Pract. 118, c165–c172. https://doi.org/10.1159/000321438 (2010).
Chen, J.-H. et al. Early elimination of uremic toxin ameliorates AKI-to-CKD transition. Clin. Sci. 135, 2643–2658. https://doi.org/10.1042/cs20210858 (2021).
Gharaie, S. et al. Microbiome modulation after severe acute kidney injury accelerates functional recovery and decreases kidney fibrosis. Kidney Int. 104, 470–491. https://doi.org/10.1016/j.kint.2023.03.024 (2023).
Murad, Y. M. et al. False negative results in clostridium difficile testing. BMC Infect. Dis. 16, 430. https://doi.org/10.1186/s12879-016-1741-6 (2016).
Peterson, C. T., Sharma, V., Elmén, L. & Peterson, S. N. Immune homeostasis, dysbiosis and therapeutic modulation of the gut microbiota. Clin. Exp. Immunol. 179, 363–377. https://doi.org/10.1111/cei.12474 (2015).
Qin, X., Caputo, F. J., Xu, D. Z. & Deitch, E. A. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock 29, 372–376. https://doi.org/10.1097/shk.0b013e3181453f4e (2008).
Chung, S., Barnes, J. L. & Astroth, K. S. Gastrointestinal microbiota in patients with chronic kidney disease: A systematic review. Adv. Nutr. 10, 888–901. https://doi.org/10.1093/advances/nmz028 (2019).
Mahmoodpoor, F., Rahbar Saadat, Y., Barzegari, A., Ardalan, M. & Zununi Vahed, S. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 93, 412–419. https://doi.org/10.1016/j.biopha.2017.06.066 (2017).
Adelman, M. W. et al. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care 24, 278. https://doi.org/10.1186/s13054-020-02989-1 (2020).
Luo, D. et al. The effects of hemodialysis and peritoneal dialysis on the gut microbiota of end-stage renal disease patients, and the relationship between gut microbiota and patient prognoses. Front. Cell Infect. Microbiol. 11, 579386. https://doi.org/10.3389/fcimb.2021.579386 (2021).
LaPlante, K. L., Dhand, A., Wright, K. & Lauterio, M. Re-establishing the utility of tetracycline-class antibiotics for current challenges with antibiotic resistance. Ann. Med. 54, 1686–1700. https://doi.org/10.1080/07853890.2022.2085881 (2022).
Talpaert, M. J., Gopal Rao, G., Cooper, B. S. & Wade, P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J. Antimicrob. Chemother. 66, 2168–2174. https://doi.org/10.1093/jac/dkr253 (2011).
Liu, J., Xie, H., Ye, Z., Li, F. & Wang, L. Rates, predictors, and mortality of sepsis-associated acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. 21, 318. https://doi.org/10.1186/s12882-020-01974-8 (2020).
Ishikawa, K. Severe acute kidney injury associated with giardia lamblia infection. Am. J. Trop. Med. Hyg. 109, 4–5. https://doi.org/10.4269/ajtmh.23-0075 (2023).
He, F.-F. et al. Sepsis-induced AKI: From pathogenesis to therapeutic approaches. Front. Pharmacol. https://doi.org/10.3389/fphar.2022.981578 (2022).
Stevens, D. L. The role of vancomycin in the treatment paradigm. Clin. Infect. Dis. 42, S51–S57 (2006).
Delaloye, J. & Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 5, 161–169. https://doi.org/10.4161/viru.26187 (2014).
Freitas, C. G. & Felipe, M. S. Candida albicans and antifungal peptides. Infect. Dis. Ther. 12, 2631–2648. https://doi.org/10.1007/s40121-023-00889-9 (2023).
Johnson, A. E. W. et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci. Data 10, 1. https://doi.org/10.1038/s41597-022-01899-x (2023).
Shankar-Hari, M. et al. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315, 775–787. https://doi.org/10.1001/jama.2016.0289 (2016).
Kellum, J. A. et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Zhang, Z., Gayle, A. A., Wang, J., Zhang, H. & Cardinal-Fernandez, P. Comparing baseline characteristics between groups: An introduction to the CBCgrps package. Ann. Transl. Med. 5, 484 (2017).
Acknowledgements
The patients participating in the study are acknowledged for their cooperation.
Author information
Authors and Affiliations
Contributions
Yaoyuan Cao: Statistical analysis and writing the paper. Fuxing Deng: Study design, paper editing and overall study supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cao, Y., Deng, F. Positive pathogens in stool could predict the clinical outcomes of sepsis-associated acute kidney injury in critical ill patient. Sci Rep 14, 11227 (2024). https://doi.org/10.1038/s41598-024-62136-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-62136-6
Keywords
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.