Abstract
Fear conditioning leads to long-term fear memory formation and is a model for studying fear-related psychopathologies conditions such as phobias and posttraumatic stress disorder. Long-term fear memory formation is believed to involve alterations of synaptic efficacy mediated by changes in synaptic transmission and morphology in lateral amygdala (LA). EphrinA4 and its cognate Eph receptors are intimately involved in regulating neuronal morphogenesis, synaptic transmission and plasticity. To assess possible roles of ephrinA4 in fear memory formation we designed and used a specific inhibitory ephrinA4 mimetic peptide (pep-ephrinA4) targeted to EphA binding site. We show that this peptide, composed of the ephrinA4 binding domain, interacts with EphA4 and inhibits ephrinA4-induced phosphorylation of EphA4. Microinjection of the pep-ephrinA4 into rat LA 30 min before training impaired long- but not short-term fear conditioning memory. Microinjection of a control peptide derived from a nonbinding E helix site of ephrinA4, that does not interact with EphA, had no effect on fear memory formation. Microinjection of pep-ephrinA4 into areas adjacent to the amygdala had no effect on fear memory. Acute systemic administration of pep-ephrinA4 1 h after training also impaired long-term fear conditioning memory formation. These results demonstrate that ephrinA4 binding sites in LA are essential for long-term fear memory formation. Moreover, our research shows that ephrinA4 binding sites may serve as a target for pharmacological treatment of fear and anxiety disorders.
Similar content being viewed by others
Introduction
Alterations of fear have a significant role in stress and anxiety disorders in humans.1,2 While there are a number of experimental tools for studying fear and anxiety, one of the simplest and most straightforward is fear conditioning.3,4 In fear conditioning an animal associates a neutral stimulus, such as a tone, with an aversive event, typically a mild footshock.5, 6, 7, 8, 9 This paradigm is especially useful as a tool for studying the molecular basis of long-term fear memory because a putative site of memory, the lateral nucleus of the amygdala (LA), has been identified.5, 6, 7, 8, 9, 10, 11 Thus, fear conditioning provides a behavioral tool and anatomical site to assess molecular mechanisms that might mediate changes in synaptic efficacy during long-term fear memory formation and fear-related disorders, such as posttraumatic stress disorder and phobias.
Long-term fear conditioning memory (LTM) formation is believed to involve alterations of synaptic efficacy produced by modifications in neural transmission and/or structural modifications of synaptic connectivity within neuronal networks that subserve fear memory.12,13 Eph receptors and their ephrin ligands are key proteins involved in the regulation of synaptic transmission and neuronal morphology during development and in adult brain.14, 15, 16 In addition, Ephs/ephrins are involved in synaptic plasticity such as long-term potentiation (LTP), a physiological model of memory, in hippocampus17 and amygdala18—areas involved in the formation of fear memories. We are therefore interested to study whether Eph receptors and ephrins are involved in long-term fear memory formation.
In the present study we investigate the roles of ephrinA4 in fear memory formation in LA. EphrinA4 is involved in regulation of neuronal morphogenesis.19 Furthermore, it was shown that EphA4, involved in synaptic plasticity in amygdala,18 has a very high affinity (in the range of nanomolars) to ephrinA4.20 Toward that end, we designed an inhibitory ephrinA4 mimetic peptide targeted to EphA binding site. Other peptides targeted to EphA binding site were used successfully to bind EphA receptors.21,22 We further explored the effects of the ephrinA4 mimetic peptide in LA on fear memory formation and whether it may serve as a tool for pharmacological treatment of fear-related disorders by injecting it systemically.
Materials and methods
Animals
Male Sprague–Dawley rats (250–300 g), were used in the study (Harlan Laboratories, Jerusalem, Israel). Following surgery, the rats were housed separately at 22±2 °C in a 12 h light/dark cycle, with free access to food and water. Behavioral experiments were approved by the University of Haifa Institutional Committee for animal experiments in accordance with National Institutes of Health guidelines.
Surgical procedures
Rats were anesthetized with xylazine 2% (15 mg per kg) and ketamine 100 mg per ml (120 mg per kg). Calmagine (Vetoquinol) (0.01 ml) was injected for analgesia before surgery. Guide stainless-steel cannulas (23 gauge) were implanted bilaterally 1.5 mm above the LA (LA coordinates are in reference to bregma: anteroposterior (AP), −3.0; lateral (L) ±5.2; and dorsoventral (DV), −8.0). Following surgery, the rats received antibiotics (0.25 ml; Pen and Strep, Norbrook, Newry, Northern Ireland). The animals recovered for 5–7 days before behavioral training.
Microinjection
The stylus was removed from the guide cannula and a 28-gauge injection cannula, extending 1.5 mm from the tip of the guide cannula aimed to the LA was carefully placed. The injection cannula was connected via PE20 tubing, back filled with saline with a small air bubble separating the saline from the peptide solution, to a 10 μl Hamilton micro-syringe, driven by a microinjection pump (CMA/100, Carnegie Medicine; or PHD 2000, Harvard Apparatus, Holliston, MA, USA). Solution was injected at a rate of 0.5 μl per min. Total volume injected per amygdala was 0.5 μl. Pep-ephrinA4 peptide (Ac-RRQRYTPFPLGFE-Lys(biotin), GL Biochem, Shanghai, China) and control peptide (Ac-RRWSGYEACTAEG-Lys(biotin); GL Biochem) were dissolved in saline at a concentration of 10 μg per μl. Following injection, the injection cannula was left for an additional 1 min before withdrawal to minimize dragging of injected liquid along the injection track.
Fear conditioning
Rats were habituated for 2 days to the training chamber for 30 min each day and briefly to the injection machine. On the next day the animals were subjected to the fear conditioning protocol. Three hundred seconds after the start of the training, animals were presented with five pairings of tone (conditioned stimulus (CS)—40 s, 5 kHz, 80 dB) that co-terminated with a foot shock (unconditioned stimulus (US)—0.5 s, 1.5 mA). The inter-trial interval was random with an average of 180 s. Rat groups were tested in a different context 1 h after training for short-term memory or 24 h after training for long-term memory. Three hundred seconds after the start of testing, animals were subjected to five tone presentations (40 s, 5 kHz, 80 dB) with inter-trial interval of 180 s in average.
Acute systemic administration of pep-ephrinA4
Rats were trained for fear conditioning as above, and 1 h after training, were injected subcutaneously with pep-ephrinA4 (0.2 mg of pep-ephrinA4 in saline) or saline. Animals were tested for long-term fear memory as above.
Histology
After behavior was completed, the rats were killed and brains were quickly removed, placed on dry ice and stored at −80 °C until use. Brains were sliced (50 μm) and stained with cresyl violet acetate to verify cannula placements.
Pull-down assay
Rats brains were homogenized with Tris lysis buffer (50 mM tris, 1% NP40, 2 mM EDTA and protease inhibitors). Biotynilated pep-ephrinA4 (20 μg) or pep-control (20 μg) were incubated for 1 h with equal amount of brain lysate diluted with 50 mM Tris buffer at room temperature followed by incubation for 1 h with 50 μl Streptavidin Agarose (Invitrogen, Carlsbad, CA, USA). The samples were washed with 50 mM Tris buffer. Proteins were eluted with boiled sample buffer, separated in an SDS–polyacrylamide gel electrophoresis and EphA4, EphB2 or eEF2 proteins levels were tested by immunoblotting. Blots were blocked with blocking buffer (5% nonfat dry milk in wash buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween-20)) for 1 h at room temperature. Blots were then subjected to purified mouse anti-EphA4/Sek antibody (1:3000—BD Transduction Laboratories, San Jose, CA, USA), rabbit eEF2 antibody (1:1000—Cell Signaling, Danvers, MA, USA) or EphB2 (1:3000; Millipore, Temecula, CA, USA) in blocking buffer for 1 h at room temperature. The blots were subjected to HRP-conjugated anti-mouse or anti-rabbit IgG (1:5000; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in wash buffer for 1 h at room temperature. Blots were washed with wash buffer for 30 min. The blots were subjected to ECL for 1 min and visualized and quantified using a CCD camera (XRS; Bio-Rad, Hercules, CA, USA). The labeled protein bands in immunoblots were analyzed using the Quantity One software.
EphrinA4 stimulation and EphA4 tyrosine phosphorylation measurements
Slice preparation: Rats were anesthetized (Pental, 0.1–0.2 mg per 100 g, CTS) and killed by decapitation. The brains were quickly removed. Immediately after, coronal brain slices (300 μm), that include the amygdala, were prepared using a vibratome (the chamber was filled with ice-cold oxygenated (95% O2, 5% CO2) Ringer’s solution (in mM: 124 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2 and 10 glucose)). The slices were then incubated in oxygenated Ringer’s solution at room temperature for 1 h to recover from the preparation procedure. EphrinA4 stimulation: After 1 h recovery the amygdala slices were incubated with oxygenated Ringer's solution or oxygenated Ringer’s solution with the pep-ephrinA4 (0.4 μg per μl) for 20 min at room temperature followed by the application of ephrinA4-Fc (4 μg per ml; R&D Systems, Minneapolis, MN, USA) for an additional 20 min at room temperature. Additional group of control slices was left in the oxygenated Ringer’s solution. After treatment, the slices were kept at −80 °C until use. Immunoprecipitation: Slices were lysed with lysis buffer (50 mM Tris, 2 mM EDTA, 1% NP40, antiproteases (Sigma, St. Louis, MO, USA) and antiphosphatases (Sigma). Twenty microliters of protein G plus/Protein A agarose beads (Calbiochem, Billerica, MA, USA) and 2 μl (0.4 μg) of anti-EphA4 (S-20, Santa Cruz Biotechnology, Dallas, TX, USA) were added to the slice lysate and incubated with shaking for 3 h at 4 °C. The beads were washed twice with phosphate-buffered saline. Forty microliters of sample buffer was added to the beads and the mixture was heated to 80 °C for 5 min to elute the proteins. The protein extract was subjected to WB procedure. Blots were blocked with blocking buffer (5% nonfat dry milk in wash buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween-20)) for 1 h at room temperature. The blots were probed with anti-phosphotyrosine antibody (1:2000, Millipore) or with antibody to the Eph4 receptors (1:2000, BD Transduction Laboratories, San Jose, CA, USA) overnight at 4 °C. The blots were washed 3 times (10 min each wash) with phosphate-buffered saline with Tween-20. The blots were subjected to a secondary anti-IgG anti-mouse peroxidase-conjugated antibody (1:5000; Jackson Immunoresearch Laboratories) for 1 h at room temperature. Blots were washed thrice (10 min each wash) in wash buffer. The blots were subjected to ECL for 1 min and visualized and quantified using a CCD camera (XRS; Bio-Rad). The labeled protein bands in immunoblots were analyzed using the Quantity One software. Background was subtracted from the measured band. The level of phospho-EphA4 was normalized to EphA4 level in the precipitate. To enable a comparison between the three groups across experiments, we normalized the signals by dividing the signal obtained above (phospho-EphA4/EphA4) in each individual slice taken from the ephrinA4, ephrinA4+pep-ephrinA4 or control (Ringer’s solution) groups by the average signal (phospho-EphA4/EphA4) value of the ephrinA4 group.
Molecular modeling
The molecular modeling of ephrinA4 was built using the Swiss-Model. Molecular graphics and superposition were performed using Pymol (http://www.pymol.org/). From the ephrinA4 model, we used the GH flexible loop involved in the binding process to design the inhibitor peptide: RRQRYTPFPLGFE. For negative control we used a peptide derived from the ephrinA4 E helix: RRWSGYEACTAEG.
Statistics
All experiments were statistically analyzed using SPSS. Behavioral analyses were performed using repeated measures analysis of variance followed by Tukey’s HSD post hoc test. For the biochemical experiment, one-way analysis of variance was conducted comparing the experimental groups followed by least significant difference post hoc test. Differences were considered significant if P<0.05.
Results
EphrinA4 peptide binds EphA4 and inhibits ephrinA4-induced EphA4 phosphorylation
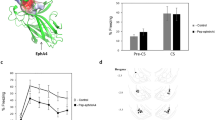
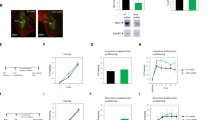
To study the roles of ephrinA4 in fear conditioning we designed and used an ephrinA4 peptide targeted to EphA binding site (pep-ephrinA4). The pep-ephrinA4 was designed to mimic the ephrinA4 binding domain (GH loop) according to the structure configuration provided by a molecular model (Figure 1a). A control peptide (pep-control) was derived from a nonbinding site of ephrinA4 (E helix, Figure 1a). Pull-down experiments of rat brain homogenates show that the pep-ephrinA4 binds the EphA4 receptor whereas the pep-control or agarose beads alone do not (Figure 1b). The analysis of variance analysis showed a significant effect for group (F(2)=4.513, P<0.05), and post hoc analysis showed that more EphA4 was pulled down in the pep-ephrinA4 group than in the pep-control (P<0.04) or beads (P<0.03). These results show that the pep-ephrinA4 interacts with EphA4 whereas the pep-control does not. Pep-ephrinA4 did not bind EphB2 receptor and the elongation factor 2 (eEF2) that served as control proteins (Figure 1b).
EphrinA4 mimetic peptide interacts with EphA4 receptor and inhibits ephrinA4-induced EphA4 phosphorylation. (a) A molecular model of ephrinA4 binding to EphA4 receptor using the Swiss-Model. Upper figure: The ephrinA4 peptide (pep-ephrinA4) is derived from the GH loop binding domain of ephrinA4 (arrow-magenta). The control peptide (pep-control) is derived from E helix of the ephrinA4 (arrow-yellow). Lower figure: A molecular model of pep-ephrinA4 peptide bound to EphA4. (b) Pull-down experiments show that pep-ephrinA4, but not pep-control or agarose beads alone, interacts with EphA4. The analysis of variance (ANOVA) showed a significant effect for group (F(2)=4.513, P<0.05), and post hoc analysis found that more EphA4 was pulled down in the pep-ephrinA4 group (n=4) than in the pep-control (P<0.04; n=3) or beads (P<0.03; n=4). Pep-ephrinA4 did not interact with the EphB2 receptor or elongation factor 2 protein (eEF2) serving as control proteins. (c) Pep-ephrinA4 inhibits ephrinA4-induced EphA4 tyrosine phosphorylation. Brain slices that contain the amygdala were divide to three groups: (1) placed in Ringer’s solution, (2) placed in Ringer’s solution and stimulated with ephrinA4-Fc for 20 min or (3) placed in Ringer’s solution with pep-ephrinA4 for 20 min followed by stimulation with ephrinA4-Fc for 20 min (n=3 each). Protein extracts from the slices were immunoprecpitated with anti-EphA4 antibody and subjected to western blot with anti-EphA4 or anti-phosphotyrosine antibodies. The upper panel shows that pep-ephrinA4 abolished ephrinA4-induced EphA4 receptor tyrosine phosphorylation. The lower panel shows the EphA4 protein level in immunoprecipitates. The ANOVA analysis showed a significant effect for group (F(2)=5.496, P<0.05), and post hoc analysis found significant increase in EphA4 phosphorylation in the ephrinA4 group when compared with the Ringer’s solution control (P<0.05) or ephrinA4+pep-ephrinA4 (P<0.03) groups. The phosphorylation of EphA4 in the control Ringer’s group is not significantly different from the ephrinA4+pep-ephrinA4 group (P>0.6).
We then asked whether pep-ephrinA4 can inhibit ephrinA4-induced EphA4 tyrosine phosphorylation. Figure 1c shows that ephrinA4-induced tyrosine phosphorylation of EphA4, in brain slices that include the amygdala, is abolished if the slices are preincubated with the pep-ephrinA4 peptide. The analysis of variance analysis found significant effect for group (F(2)=5.496, P<0.05), and post hoc analysis showed that significantly more phosphorylation is detected in EphA4 receptors in the ephrinA4 group than in the Ringer’s solution control (P<0.05) or ephrinA4+pep-ephrinA4 (P<0.03) groups. The phosphorylation of EphA4 in the control Ringer’s group is not significantly different from the ephrinA4+pep-ephrinA4 group (P>0.6). This result shows that pep-ephrinA4 can inhibit ephrinA4-induced EphA4 phosphorylation.
Pep-ephrinA4 peptide in LA impairs LTM formation
We hypothesize that ephrinA4 interaction with EphA is essential for fear conditioning memory formation in LA and that microinjection of the inhibitory pep-ephrinA4 peptide into this brain area will interfere with the process of creating long-term fear memory. To test this hypothesis we compared the effects of the pep-ephrinA4 to that of the pep-control, that do not interact with EphA, on long-term fear memory formation in LA. We microinjected the pep-ephrinA4, pep-control or saline into the LA 30 min before fear conditioning. Rats with cannula tips at or within the boundaries of lateral and basal amygdala (LBA) were included in the data analysis (Figure 2). Long-term conditioned fear memory was assessed by measuring freezing responses elicited by the CS without the unconditioned stimulus 24 h after conditioning. Figure 2a shows that rats microinjected with pep-ephrinA4 froze significantly less (F(3)=6.428, P<0.003) than animals injected with saline (P<0.02), control peptide (P<0.005) or animals injected with pep-ephrinA4 in areas adjacent to the LA (P<0.03), showing that pep-ephrinA4-treated animals are impaired in long-term fear memory. The group × tone trial interaction did not differ significantly (F(8.481)=1.487, P>0.17), indicating that pep-ephrinA4 did not alter the rate of fear reduction over the trials when compared with controls. The control microinjected groups (saline, control peptide and misplaced pep-ephrinA4) were not different from each other (P>0.5). Cumulatively, these results show that microinjection of the ephrinA4 inhibitory pep-ephrinA4 peptide into LA impairs LTM.
Inhibitory ephrinA4 mimetic peptide in the lateral amygdala (LA) impairs long-term fear memory (LTM) formation. (a) Pep-ephrinA4 (0.5 μl of 10 μg per μl; n=9), pep-control (0.5 μl of 10 μg per μl; n=5) or saline (n=7) were microinjected into rat LA 30 min before fear conditioning. Long-term memory was tested 24 h after training. Animals injected with pep-ephrinA4 were significantly impaired in fear memory (F(3)=6.428, P<0.003) when compared with pep-control (P<0.005), saline (P<0.02) or pep-ephrinA4 injected into areas surrounding the LA (P<0.03, n=11). The control microinjected groups (saline, control peptide and misplaced pep-ephrinA4) were not different from each other (P>0.5). (b) Cannula placements within the boundaries of lateral and basal amygdala (LBA) of pep-ephrinA4-, saline- and pep-control-microinjected rats. (c) Cannula placements of pep-ephrinA4 microinjected in areas near LA. CS, conditioned stimulus.
Pep-ephrinA4 in LA has no effect on short-term fear conditioning memory formation
To obtain additional insights into the involvement of ephrinA4 binding in LA in fear memory formation we were interested to study whether microinjection of the inhibitory pep-ephrinA4 mimetic peptide into LA will affect short-term fear memory. Interference with short-term fear memory formation may infer that ephrinA4 binding affects memory acquisition whereas specific involvement in LTM formation implies that ephrinA4 binding is needed for fear memory consolidation. We microinjected the pep-ephrinA4 30 min before fear conditioning into rats LA and compared their freezing 1 h after training to freezing of saline-microinjected animals. Repeated measures analysis revealed no main effect for groups (F(1)=0.712; P>0.4, Figure 3a). The group × tone trial interaction was not significant (F(12)=0.336, P>0.8). These results show that ephrinA4 is not involved in the formation of short-term fear memory. In addition, freezing before the training (pre-CS) or during tones in training was not affected by the treatment (Pre-CS, t-test P>0.252; (F(1)=0.938; P>0.3); pep-ephrinA4 (n=7); saline (n=8); Figure 3b). The treatment × tone trial interaction was not significant (F(1)=1.121; P>0.35). These results indicate that the pep-ephrinA4 do not affect freezing per se and tone and footshock processing in the LA. Cumulatively, the aforementioned findings demonstrate that the pep-ephrinA4 in LA has no effect on short-term fear memory and faculties needed for CS–US stimuli association but rather on their consolidation into LTM.
EphrinA4 mimetic peptide in the lateral amygdala (LA) has no effect on short-term fear memory (STM) formation. (a) Pep-ephrinA4 (0.5 μl of 10 μg per μl; n=8) or saline (n=8) were microinjected into the rat LA 30 min before fear conditioning. Short-term memory was tested 1 h after training. Animals injected with pep-ephrinA4 were not significantly different in fear memory (F(1)=0.712, P>0.4) when compared with the saline-treated rats. (b) Cannula placements of pep-ephrinA4 and saline-microinjected animals. (c) Freezing during training is not different between animals injected with pep-ephrinA4 and saline (F(1)=0.938; P>0.3). CS, conditioned stimulus.
Acute systemic injection of pep-ephrinA4 impairs LTM formation
The aforementioned results show that ephrinA4 binding sites may serve as a target for pharmacological treatment of fear-related disorders. Application of drugs is most useful after trauma and systemically. We therefore tested the effects of pep-ephrinA4 injected subcutaneously 1 h after fear conditioning training. As shown in Figure 4, freezing during long-term memory test in animals injected with the pep-ephrinA4 (n=15) was significantly lower than animals injected with saline (n=14; F(1)=8.6, P<0.008). The treatment × tone trial interaction was not significant (F(4)=0.939, P>0.4). These results show that systemic administration of the pep-ephrinA4 impairs fear memory formation.
Acute systemic administration of ephrinA4 mimetic peptide impairs long-term fear conditioning memory (LTM) formation. Pep-ephrinA4 (0.2 mg; n=15), or saline (n=14) was injected subcutaneously 1 h after fear conditioning. Long-term memory was tested 24 h after training. Animals injected with pep-ephrinA4 were significantly impaired in fear memory (F(1)=8.6, P<0.008) when compared with saline-injected rats. CS, conditioned stimulus.
Discussion
When fear becomes greater than that warranted by the situation, or begins to occur in inappropriate situations, a fear or anxiety disorder exists. It was shown that the fear system in the brain is involved in at least some anxiety disorders.3,4 The formation of memory is associated with alteration in synaptic transmission and morphogenesis.13 Ephrins and their cognate Eph receptors are key proteins intimately involved in regulating synaptic transmission and morphogenesis during brain development and in adults.14, 15, 16 The present study shows that microinjection of an inhibitory ephrinA4 mimetic peptide, targeted to EphA binding sites, into LA impairs LTM formation. These results indicate that ephrinA4 binding sites in LA are essential for fear memory formation. Furthermore, acute subcutaneous injection of pep-ephrinA4 1 h after fear conditioning impaired fear LTM. We cannot determine whether pep-ephrinA4 acts through the amygdala after systemic injection, but the results indicate that ephrinA4 cognate receptors may serve as potential targets for therapeutically pharmacological intervention in fear-related disorders.
Microinjection of the inhibitory ephrinA4 mimetic peptide into LA before fear conditioning impaired long- but not short-term fear conditioning memory. These results suggest that ephrinA4 binding sites in LA are needed for the consolidation of short-term memory into long-term fear memory. These observations also imply that ephrinA4 interaction is not needed for fear memory acquisition. Ephrins/Ephs are involved in the regulation of synaptic transmission.14, 15, 16 However, our results suggest that ephrinA4 sites are not needed for synaptic transmission in LA as transmission during learning is essential for fear memory acquisition.23,24
It is plausible that ephrinA4 binding mediates long-lasting neuronal alterations involved in memory consolidation in LA, rather than synaptic transmission involved in memory acquisition. LTM involves enduring alteration in molecular content in synapse and neuronal morphology.13 Ample studies show that Ephs receptors and ephrins are intimately involved in neuronal structural changes such as spine morphogenesis.14, 15, 16 It is therefore possible that the ephrinA4 is involved in such long-lasting synaptic changes. Changes in the number and shape of dendritic spines were observed following fear conditioning. For example, auditory fear conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the LA.25 Postsynaptic density area on a smooth endoplasmic reticulum-free spines increases with fear conditioning whereas the spines head volume of these spines decreases in LA.26
Ephrins and Eph receptors are also regulators of the Rho/Rac/CDC42 small GTPases that affect actin dynamics and neuronal morphology.13,27, 28, 29 Small GTPases and their affectors are involved in fear memory formation in LA.30,31 Moreover, actin cytoskeleton polymerization in LA is essential for fear memory formation.32, 33, 34, 35 It is therefore possible that ephrinA4 regulates fear memory formation in LA by controlling actin dynamics.
We observed that the pep-ephrinA4 peptide interacts with EphA4 receptor and inhibit ephrinA4-induced EphA4 phosphorylation. Interestingly, ephrinA3, another major EphA4 binding ligand, is not involved in cued fear conditioning memory formation, but rather involved in contextual memory formation,36 indicating that not all ephrinAs are involved similarly in memory formation. It would be of interest to unveil the unique properties and functions of ephrinA4 leading to its key role in memory formation in LA.
References
Marks I ed. Fears, Phobias, and Rituals: Panic, Anxiety and Their Disorders. Oxford University Press: New York, NY, USA, 1987.
Öhman A . Fear and anxiety as emotional phenomena: clinical, phenomenological, evolutionary perspectives, and information processing mechanisms. Lewis and Haviland, (eds). Handbook of Emotions. Guilford Press, 1993, pp 511–536.
Shalev AY, Forgel-Fuchs Y, Pitman RK . Conditioned fear and psychological trauma. Biol Psychiatry 1992; 31: 863–865.
Mahan AL, Ressler KJ . Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 2012; 35: 24–35.
Fanselow MS, LeDoux JE . Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 1999; 23: 229–232.
LeDoux JE . Emotion circuits in the brain. Annu Rev Neurosci 2000; 23: 155–184.
Davis M, Whalen PJ . The amygdala: vigilance and emotion. Mol Psychiatry 2001; 6: 13–34.
Sah P, Faber ES, Lopez De Armentia M, Power J . The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83: 803–834.
Maren S . Synaptic mechanisms of associative memory in the amygdala. Neuron 2005; 47: 783–786.
Schafe GE, Nader K, Blair HT, LeDoux JE . Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci 2001; 24: 540–546.
Rodrigues SM, Schafe GE, LeDoux JE . Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron 2004; 44: 75–91.
Kandel ER . The molecular biology of memory storage: a dialogue between genes and synapses. Science 2001; 294: 1030–1038.
Lamprecht R, LeDoux J . Structural plasticity and memory. Nat Rev Neurosci 2004; 5: 45–54.
Murai KK, Pasquale EB . Eph receptors, ephrins, and synaptic function. Neuroscientist 2004; 10: 304–314.
Klein R . Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci 2009; 12: 15–20.
Hruska M, Dalva MB . Ephrin regulation of synapse formation, function and plasticity. Mol Cell Neurosci 2012; 50: 35–44.
Grunwald IC, Korte M, Wolfer D, Wilkinson GA, Unsicker K, Lipp HP et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron 2001; 32: 1027–1040.
Deininger K, Eder M, Kramer ER, Zieglgänsberger W, Dodt HU, Dornmair K et al. The Rab5 guanylate exchange factor Rin1 regulates endocytosis of the EphA4 receptor in mature excitatory neurons. Proc Natl Acad Sci USA 2008; 105: 12539–12544.
Moss A, Alvares D, Meredith-Middleton J, Robinson M, Slater R, Hunt SP et al. Ephrin-A4 inhibits sensory neurite outgrowth and is regulated by neonatal skin wounding. Eur J Neurosci 2005; 22: 2413–2421.
Bowden TA, Aricescu AR, Nettleship JE, Siebold C, Rahman-Huq N, Owens RJ et al. Structural plasticity of eph receptor A4 facilitates cross-class ephrin signaling. Structure 2009; 17: 1386–1397.
Murai KK, Nguyen LN, Koolpe M, McLennan R, Krull CE, Pasquale EB et al. Targeting the EphA4 receptor in the nervous system with biologically active peptides. Mol Cell Neurosci 2003; 24: 1000–1011.
Lamberto I, Qin H, Noberini R, Premkumar L, Bourgin C, Riedl SJ et al. Distinctive binding of three antagonistic peptides to the ephrin-binding pocket of the EphA4 receptor. Biochem J 2012; 445: 47–56.
Muller J, Corodimas KP, Fridel Z, LeDoux JE . Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci 1997; 111: 683–691.
Wilensky AE, Schafe GE, LeDoux JE . Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci 1999; 19: RC48.
Radley JJ, Johnson LR, Janssen WG, Martino J, Lamprecht R, Hof PR et al. Associative Pavlovian conditioning leads to an increase in spinophilin-immunoreactive dendritic spines in the lateral amygdala. Eur J Neurosci 2006; 24: 876–884.
Ostroff LE, Cain CK, Bedont J, Monfils MH, Ledoux JE . Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci USA 2010; 107: 9418–9423.
Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell 2001; 105: 233–244.
Irie F, Yamaguchi Y . EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci 2002; 5: 1117–1118.
Penzes P, Beeser A, Chernoff J, Schiller MR, Eipper BA, Mains RE et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF kalirin. Neuron 2003; 37: 263–274.
Lamprecht R, Farb CR, LeDoux JE . Fear memory formation involves p190 RhoGAP and ROCK proteins through a GRB2-mediated complex. Neuron 2002; 36: 727–738.
Lamprecht R . The roles of the actin cytoskeleton in fear memory formation. Front Behav Neurosci 2011; 5: 39.
Mantzur L, Joels G, Lamprecht R . Actin polymerization in lateral amygdala is essential for fear memory formation. Neurobiol Learn Mem 2009; 91: 85–88.
Rehberg K, Bergado-Acosta JR, Koch JC, Stork O . Disruption of fear memory consolidation and reconsolidation by actin filament arrest in the basolateral amygdala. Neurobiol Learn Mem 2010; 94: 117–126.
Motanis H, Maroun M . Differential involvement of protein synthesis and actin rearrangement in the reacquisition of contextual fear conditioning. Hippocampus 2011; 22: 494–500.
Gavin CF, Rubio MD, Young E, Miller C, Rumbaugh G . Myosin II motor activity in the lateral amygdala is required for fear memory consolidation. Learn Mem 2011; 19: 9–14.
Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB . Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci USA 2009; 106: 12524–12529.
Acknowledgements
This work was supported by The German-Israeli Foundation for Scientific Research and Development (GIF) and Israel Science Foundation (ISF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Dines, M., Lamprecht, R. EphrinA4 mimetic peptide targeted to EphA binding site impairs the formation of long-term fear memory in lateral amygdala. Transl Psychiatry 4, e450 (2014). https://doi.org/10.1038/tp.2014.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tp.2014.76