Featured
-
-
Article |
 Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2
Mechanisms of neurotransmitter transport and drug inhibition in human VMAT2
Structures of a vesicular monoamine transporter in complex with drugs and substrate provide insights into the physiology and pharmacology of neurotransmitter packaging.
- Shabareesh Pidathala
- , Shuyun Liao
- & Chia-Hsueh Lee
-
Article |
 An amygdala circuit that suppresses social engagement
An amygdala circuit that suppresses social engagement
A circuit in the amygdala uses thyrotropin-releasing hormone to suppress male mating when a female mouse is unhealthy.
- Jeong-Tae Kwon
- , Changhyeon Ryu
- & Gloria B. Choi
-
Article |
 Structural insights into the inhibition of glycine reuptake
Structural insights into the inhibition of glycine reuptake
Serial synchrotron crystallography reveals the structure of the human glycine transporter GlyT1, showing how a state-specific inhibitor exerts its effects, and potentially informing the design of new GlyT1 inhibitors to treat a range of disorders of the central nervous system.
- Azadeh Shahsavar
- , Peter Stohler
- & Poul Nissen
-
Article |
 Glutamate transporters have a chloride channel with two hydrophobic gates
Glutamate transporters have a chloride channel with two hydrophobic gates
Glutamate transporters conduct chloride ions through an aqueous channel with hydrophobic gates that forms during the glutamate transport cycle.
- Ichia Chen
- , Shashank Pant
- & Renae M. Ryan
-
Article |
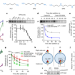 ATP13A2 deficiency disrupts lysosomal polyamine export
ATP13A2 deficiency disrupts lysosomal polyamine export
The lysosomal polyamine transporter ATP13A2 controls the cellular polyamine content, and impaired lysosomal polyamine export represents a lysosome-dependent cell death pathway that may be implicated in ATP13A2-associated neurodegeneration.
- Sarah van Veen
- , Shaun Martin
- & Peter Vangheluwe
-
Letter |
 Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport
Serotonin transporter–ibogaine complexes illuminate mechanisms of inhibition and transport
Cryo-electron microscopy reveals three conformations of the serotonin transporter in complex with ibogaine, detailing the structural rearrangements that occur between the different stages of its transport cycle.
- Jonathan A. Coleman
- , Dongxue Yang
- & Eric Gouaux
-
Article |
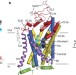 X-ray structures and mechanism of the human serotonin transporter
X-ray structures and mechanism of the human serotonin transporter
X-ray crystal structures of the human serotonin transporter (SERT) bound to the antidepressants (S)-citalopram or paroxetine show that the antidepressants lock the protein in an outward-open conformation, and directly block serotonin from entering its binding site; the structures define the mechanism of antidepressant action in SERT and pave the way for future drug design.
- Jonathan A. Coleman
- , Evan M. Green
- & Eric Gouaux
-
Research Highlights |
Flushing proteins from the brain
-
Letter |
 Structure and mechanism of a glutamate–GABA antiporter
Structure and mechanism of a glutamate–GABA antiporter
The X-ray crystal structure of the glutamate–GABA antiporter GadC is determined, revealing an inward-open conformation and providing insights into mechanism of amino acid antiport that is needed for acid resistance in bacteria.
- Dan Ma
- , Peilong Lu
- & Yigong Shi
-
Article |
 X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
The X-ray crystal structure of LeuT, the bacterial homologue of the neurotransmitter sodium symporter family, is reported in the outward-open and inward-open states.
- Harini Krishnamurthy
- & Eric Gouaux
-
Letter |
 Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue
Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue
- Yongfang Zhao
- , Daniel S. Terry
- & Jonathan A. Javitch
-
Letter |
 Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
Neurotransmitter/sodium symporter orthologue LeuT has a single high-affinity substrate site
The initial crystal structure of LeuT, together with subsequent functional and structural studies, provided direct evidence for a single, high-affinity substrate-binding site. Recent binding, flux and molecular simulation studies, however, have been interpreted in terms of a model where there are two high-affinity binding sites: the second (S2) site is believed to be located within the extracellular vestibule. Here, direct measurement is performed of substrate binding to wild-type LeuT and to S2 site mutants using isothermal titration calorimetry, equilibrium dialysis and scintillation proximity assays. The conclusion is made that LeuT harbours a single, centrally located, high-affinity substrate-binding site.
- Chayne L. Piscitelli
- , Harini Krishnamurthy
- & Eric Gouaux
-
Article |
 Single-molecule dynamics of gating in a neurotransmitter transporter homologue
Single-molecule dynamics of gating in a neurotransmitter transporter homologue
Neurotransmitter:Na+ symporters (NSS) remove neurotransmitters from the synapse in a reuptake process that is driven by the Na+ gradient. Here, single-molecule fluorescence imaging assays have been combined with molecular dynamics simulations to probe the conformational changes that are associated with substrate binding and transport by a prokaryotic NSS homologue, LeuT. The findings are interpreted in the context of an allosteric mechanism that couples ion and substrate binding to transport.
- Yongfang Zhao
- , Daniel Terry
- & Jonathan A. Javitch

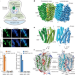 Structural insights into vesicular monoamine storage and drug interactions
Structural insights into vesicular monoamine storage and drug interactions