Featured
-
-
Article |
 NMDA receptor structures reveal subunit arrangement and pore architecture
NMDA receptor structures reveal subunit arrangement and pore architecture
X-ray crystal structures are presented of the N-methyl-d-aspartate (NMDA) receptor, a calcium-permeable ion channel that opens upon binding of glutamate and glycine; glutamate is a key excitatory neurotransmitter and enhanced structural insight of this receptor may aid development of therapeutic small molecules.
- Chia-Hsueh Lee
- , Wei Lü
- & Eric Gouaux
-
News & Views |
 Lipopolysaccharide rolls out the barrel
Lipopolysaccharide rolls out the barrel
Two crystal structures of the LptD–LptE protein complex reveal how the cell-wall component lipopolysaccharide is delivered and inserted into the external leaflet of the bacterial outer membrane. See Article p.52 & Letter p.108
- Russell E. Bishop
-
News & Views |
 Enzyme assembly line pictured
Enzyme assembly line pictured
Many enzymes form 'assembly lines' containing a series of catalytic modules. Visualization of how the structure of a module shifts during catalysis provides a clearer idea of how such enzymes work. See Article p.512 & Letter p.560
- Peter F. Leadlay
-
Letter |
 Structural basis for lipopolysaccharide insertion in the bacterial outer membrane
Structural basis for lipopolysaccharide insertion in the bacterial outer membrane
Lipopolysaccharide, an essential component of the Gram-negative bacteria outer membrane, is inserted by LptD–LptE, a protein complex with a unique ‘barrel and plug’ architecture; the structure of the LptD–LptE complex of Shigella flexneri determined here shows LptD forming a 26-stranded β-barrel with LptE located inside the barrel of LptD, the first two β-strands are distorted by two proline residues, creating a potential portal in the barrel wall that might allow lateral diffusion of lipopolysaccharide into the outer membrane.
- Shuai Qiao
- , Qingshan Luo
- & Yihua Huang
-
Article |
 Structural basis for outer membrane lipopolysaccharide insertion
Structural basis for outer membrane lipopolysaccharide insertion
Lipopolysaccharide, an essential component of the outer membranes of Gram-negative bacteria, is inserted by LptD–LptE, a protein complex with a unique ‘barrel and plug’ architecture; the structure, molecular dynamics simulations and functional assays of the LptD–LptE complex of Salmonella typhimurium suggest that lipopolysaccharide may pass through the barrel and is then inserted into the outer leaflet of the outer membrane through a lateral opening between two β-strands of LptD.
- Haohao Dong
- , Quanju Xiang
- & Changjiang Dong
-
Letter |
 Structural rearrangements of a polyketide synthase module during its catalytic cycle
Structural rearrangements of a polyketide synthase module during its catalytic cycle
Polyketide synthases (PKSs) are multidomain enzymes that produce polyketides, which form the basis of many therapeutic agents; here, electron cryo-microscopy is used to probe the structure of an intact module of a multi-enzyme PKS in different functional states.
- Jonathan R. Whicher
- , Somnath Dutta
- & Georgios Skiniotis
-
Article |
 Structure of a modular polyketide synthase
Structure of a modular polyketide synthase
Polyketide synthases are multidomain enzymes that produce polyketides, which form the basis of many therapeutic agents; here, electron cryo-microscopy is used to establish the structure of a bacterial full-length module, and to elucidate the structural basis of both intramodule and intermodule substrate transfer.
- Somnath Dutta
- , Jonathan R. Whicher
- & Georgios Skiniotis
-
News & Views |
 Wobble puts RNA on target
Wobble puts RNA on target
Enzymes that attach amino acids to transfer RNAs during protein synthesis must recognize both substrates specifically. Crystal structures reveal a mechanism that explains the RNA specificity for one such system. See Article p.507
- Oscar Vargas-Rodriguez
- & Karin Musier-Forsyth
-
Article |
 The selective tRNA aminoacylation mechanism based on a single G•U pair
The selective tRNA aminoacylation mechanism based on a single G•U pair
X-ray crystal structures of a tRNA synthetase bound to wild-type and mutant alanine tRNAs reveal the structural basis for selectivity.
- Masahiro Naganuma
- , Shun-ichi Sekine
- & Shigeyuki Yokoyama
-
Article |
 Crystal structure of a human GABAA receptor
Crystal structure of a human GABAA receptor
GABAA receptors are the principal mediators of rapid inhibitor synaptic transmission in the brain, and a decline in GABAA signalling leads to diseases including epilepsy, insomnia, anxiety and autism; here, the first X-ray crystal structure of a human GABAA receptor, the human β3 homopentamer, reveals structural features unique for this receptor class and uncovers the locations of key disease-causing mutations.
- Paul S. Miller
- & A. Radu Aricescu
-
Letter |
 The structural basis of transfer RNA mimicry and conformational plasticity by a viral RNA
The structural basis of transfer RNA mimicry and conformational plasticity by a viral RNA
RNA molecules can perform multiple functions, which can be driven by different conformational states; here, the crystal structure of the transfer-RNA-like structure of the turnip yellow mosaic virus is solved, providing insight into the structural basis of RNA multifunctionality.
- Timothy M. Colussi
- , David A. Costantino
- & Jeffrey S. Kieft
-
Letter |
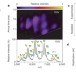 Membrane proteins bind lipids selectively to modulate their structure and function
Membrane proteins bind lipids selectively to modulate their structure and function
A new mass-spectrometry method has been developed to obtain high-resolution spectra of folded proteins bound to lipids; using this technique as well as X-ray crystallography provides evidence for membrane protein conformational change as a result of lipid–protein interaction.
- Arthur Laganowsky
- , Eamonn Reading
- & Carol V. Robinson
-
Article |
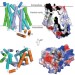 Crystal structure of the human glucose transporter GLUT1
Crystal structure of the human glucose transporter GLUT1
The structure of human GLUT1 in an inward-open conformation is reported; access to the structure of the human protein, instead of just a bacterial homologue, made it possible to map (inactivating) mutations associated with GLUT1 deficiency syndrome onto the structure.
- Dong Deng
- , Chao Xu
- & Nieng Yan
-
Editorial |
Hard data
It has been no small feat for the Protein Data Bank to stay relevant for 100,000 structures.
-
Article |
 Structural basis of the non-coding RNA RsmZ acting as a protein sponge
Structural basis of the non-coding RNA RsmZ acting as a protein sponge
A novel combined NMR and EPR spectroscopy approach reveals the structure and assembly mechanism of a 70-kDa bacterial ribonucleoprotein complex acting as a protein sponge in translational regulation.
- Olivier Duss
- , Erich Michel
- & Frédéric H.-T. Allain
-
Letter |
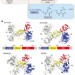 Ribosomal oxygenases are structurally conserved from prokaryotes to humans
Ribosomal oxygenases are structurally conserved from prokaryotes to humans
Crystal structures of human and prokaryotic ribosomal oxygenases reported here, with and without their ribosomal protein substrates, support their assignments as hydroxylases, and provide insights into the evolution of the JmjC-domain-containing hydroxylases and demethylases.
- Rasheduzzaman Chowdhury
- , Rok Sekirnik
- & Christopher J. Schofield
-
Letter |
 A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome
A Ctf4 trimer couples the CMG helicase to DNA polymerase α in the eukaryotic replisome
This study shows how the yeast Ctf4 protein couples the DNA helicase, Cdc45–MCM–GINS, to DNA polymerase α — the GINS subunit of the helicase and the polymerase use a similar interaction to bind Ctf4, suggesting that, as Ctf4 is a trimer, two polymerases could be simultaneously coupled to a single helicase during lagging-strand synthesis.
- Aline C. Simon
- , Jin C. Zhou
- & Luca Pellegrini
-
News & Views |
 Action at a distance in a light receptor
Action at a distance in a light receptor
A tour de force of X-ray scattering has yielded structures of a phytochrome photoreceptor in its dark and illuminated states, showing how localized protein refolding magnifies a light signal to form a cellular message. See Letter p.245
- Anna W. Baker
- & Katrina T. Forest
-
Letter |
 Signal amplification and transduction in phytochrome photosensors
Signal amplification and transduction in phytochrome photosensors
The solution and crystal structures of a bacterial phytochrome photosensory core in both its resting and activated states are determined; switching between closed (resting) and open (activated) forms is found to be mediated by a conserved ‘tongue’, and the structures indicate that smaller changes in the vicinity of the chromophore are amplified in scale as they are transmitted through the tongue and beyond.
- Heikki Takala
- , Alexander Björling
- & Sebastian Westenhoff
-
Letter |
 Agonist-bound structure of the human P2Y12 receptor
Agonist-bound structure of the human P2Y12 receptor
An X-ray structure of human P2Y12 receptor, a clinical drug target for platelet aggregation inhibitors, is presented in complex with an agonist, providing insight into the δ-group of class A G-protein-coupled receptors.
- Jin Zhang
- , Kaihua Zhang
- & Qiang Zhao
-
Letter |
 Structure of the AcrAB–TolC multidrug efflux pump
Structure of the AcrAB–TolC multidrug efflux pump
Many bacteria are able to survive in the presence of antibiotics in part because they possess pumps that can remove a broad range of small molecules; here, the structure of one such pump, AcrAB–TolC, is determined using X-ray crystallography and cryo-electron microscopy.
- Dijun Du
- , Zhao Wang
- & Ben F. Luisi
-
Letter |
 Structural basis of Sec-independent membrane protein insertion by YidC
Structural basis of Sec-independent membrane protein insertion by YidC
The crystal structure of the bacterial protein YidC is reported, together with a structure-based functional analysis, providing insight into the role of YidC in inserting single-spanning membrane proteins into the membrane.
- Kaoru Kumazaki
- , Shinobu Chiba
- & Osamu Nureki
-
-
News & Views |
 The purple heart of photosynthesis
The purple heart of photosynthesis
The structure of a photosynthetic complex from a purple bacterium reveals a new class of light-harvesting protein and the channels that might allow electron-transporting molecules to escape this otherwise closed system. See Article p.228
- Richard J. Cogdell
- & Aleksander W. Roszak
-
Article |
 Structure of the LH1–RC complex from Thermochromatium tepidum at 3.0 Å
Structure of the LH1–RC complex from Thermochromatium tepidum at 3.0 Å
The near-atomic-level structure of a complete bacterial light-harvesting antenna–reaction centre (LH1–RC) complex is described here; the structure reveals how energy is transferred from the LH1 to the RC in a highly efficient way and suggests how ubiquinone might cross a closed LH1 barrier.
- Satomi Niwa
- , Long-Jiang Yu
- & Kunio Miki
-
Letter |
 Structure of the human P2Y12 receptor in complex with an antithrombotic drug
Structure of the human P2Y12 receptor in complex with an antithrombotic drug
The X-ray crystal structure of the human P2Y12 receptor, which regulates platelet activation and thrombus formation, is solved in complex with an antithrombotic drug, providing insights for the development of new drugs.
- Kaihua Zhang
- , Jin Zhang
- & Qiang Zhao
-
Letter |
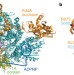 Structural basis for translocation by AddAB helicase–nuclease and its arrest at χ sites
Structural basis for translocation by AddAB helicase–nuclease and its arrest at χ sites
A dual-function helicase–nuclease, typified by RecBCD in Escherichia coli, acts on free DNA ends during bacterial double-stranded break repair until it reaches a χ sequence at which it pauses before continuing with modified enzymatic properties; here several crystal structures of the related AddAB enzyme from Bacillus subtilis bound to χ-containing DNA are presented, offering insight into χ recognition and its effect on DNA translocation.
- Wojciech W. Krajewski
- , Xin Fu
- & Dale B. Wigley
-
Letter |
 Interrogating selectivity in catalysis using molecular vibrations
Interrogating selectivity in catalysis using molecular vibrations
A set of parameters based on the response of a molecule’s properties to infrared vibrations can be used to model and predict selectivity trends for molecular reactions with interlinked steric and electronic effects at positions of interest
- Anat Milo
- , Elizabeth N. Bess
- & Matthew S. Sigman
-
Letter |
 Structure of a type IV secretion system
Structure of a type IV secretion system
The three-dimensional structure of the type IV secretion system encoded by the Escherichia coli R388 conjugative plasmid.
- Harry H. Low
- , Francesca Gubellini
- & Gabriel Waksman
-
Letter |
 ZMYND11 links histone H3.3K36me3 to transcription elongation and tumour suppression
ZMYND11 links histone H3.3K36me3 to transcription elongation and tumour suppression
Candidate tumour suppressor ZMYND11 specifically recognizes histone K36 trimethylation on the histone variant H3.3 and helps regulate transcription elongation.
- Hong Wen
- , Yuanyuan Li
- & Xiaobing Shi
-
Letter |
 Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I
Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I
RIG-I protein recognizes viral duplex RNA with a 5′-triphosphate group, activating innate immune responses; a crystal structure of its tetrameric CARD signalling domain reveals that non-covalently linked ubiquitin chains stabilize the tetramer in a ‘lock-washer’ structure that serves as a signalling platform for the recruitment and activation of MAVS.
- Alys Peisley
- , Bin Wu
- & Sun Hur
-
News & Views |
 How to switch affinity
How to switch affinity
The protein NRT1.1 transports nitrate ions into plants over a wide range of concentrations. Two studies provide structural insight into this unusual behaviour, but give different explanations for it. See Articles p.68 & p.73
- Yi-Fang Tsay
-
Article |
 Crystal structure of the plant dual-affinity nitrate transporter NRT1.1
Crystal structure of the plant dual-affinity nitrate transporter NRT1.1
A description of the crystal structure of unphosphorylated NRT1.1 provides insights into how phosphorylation switches the nitrate transporter between the low-affinity and high-affinity states.
- Ji Sun
- , John R. Bankston
- & Ning Zheng
-
Article |
 Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1
Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1
In Arabidopsis thaliana the phosphorylation state of the ‘dual affinity’ transporter, NRT1.1, allows the uptake of nitrate over a wide concentration range; the crystal structure and molecular basis for this is described in this study.
- Joanne L. Parker
- & Simon Newstead
-
Article |
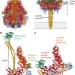 Mechanism of Tc toxin action revealed in molecular detail
Mechanism of Tc toxin action revealed in molecular detail
High-resolution structures of the Photorhabdus luminescens TcA toxin subunit and the entire Tc toxin complex reveal important new insights into Tc complex structure and function.
- Dominic Meusch
- , Christos Gatsogiannis
- & Stefan Raunser
-
Letter |
 Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2
Structure of the core ectodomain of the hepatitis C virus envelope glycoprotein 2
The crystal structure of the core domain of the hepatitis C virus surface glycoprotein E2 has been solved; the structure shows that, contrary to expectation, E2 is unlikely to be the viral fusion protein.
- Abdul Ghafoor Khan
- , Jillian Whidby
- & Joseph Marcotrigiano
-
Research Highlights |
Enzyme shifts shape to edit DNA
-
Letter |
 Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion
Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion
Nascent secretory and membrane proteins are targeted to the Sec61 protein-conducting channel for translocation across or insertion into the endoplasmic reticulum membrane; here cryo-electron microscopy structures of eukaryotic ribosome–channel complexes show how this channel opens vertically during translocation of a secretory protein into the lumen of the endoplasmic reticulum and how it opens laterally during insertion of a transmembrane domain into the lipid bilayer.
- Marko Gogala
- , Thomas Becker
- & Roland Beckmann
-
Article |
 Proof of principle for epitope-focused vaccine design
Proof of principle for epitope-focused vaccine design
Computational protein design methods are used to generate new candidates for a human respiratory syncytial virus (RSV) vaccine; artificial protein scaffolds that mimic the structure of a RSV epitope are shown to induce RSV-specific neutralizing antibodies in macaques.
- Bruno E. Correia
- , John T. Bates
- & William R. Schief
-
Comment |
 History: Women in crystallography
History: Women in crystallography
Georgina Ferry celebrates the egalitarian, collaborative culture that has so far produced two female Nobel prizewinners.
- Georgina Ferry
-
News & Views |
 Sources of inspiration
Sources of inspiration
Synchrotrons have long been the preferred X-ray sources for crystallography, but competition has arrived with the advent of X-ray free-electron lasers. A synchrotron expert and an advocate of free-electron lasers discuss the prospects of the respective source types for applications in structural biology.
- Sean McSweeney
- & Petra Fromme
-
Letter |
 A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes
A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes
A genetic locus from the gut symbiont Bacteroides ovatus is identified and described that encodes a cohort of enzymes and carbohydrate-binding proteins necessary for the metabolism of xyloglucans—a predominant component of dietary fibre.
- Johan Larsbrink
- , Theresa E. Rogers
- & Harry Brumer
-
Article |
 Molecular control of δ-opioid receptor signalling
Molecular control of δ-opioid receptor signalling
The 1.8 Å high-resolution X-ray crystal structure of the human δ-opioid receptor is presented, with site-directed mutagenesis and functional studies revealing a crucial role for a sodium ion in mediating allosteric control in this receptor.
- Gustavo Fenalti
- , Patrick M. Giguere
- & Raymond C. Stevens
-
Research Highlights |
Better pictures of protein structures
-
News & Views |
 Ringside views
Ringside views
Two crystal structures reveal that the Vif and Vpx proteins of human and simian immunodeficiency viruses mediate evasion of host defences by reprogramming the cellular protein-degradation machinery. See Letters p.229 & p.234
- Michael H. Malim
-
Letter |
 Structural basis for hijacking CBF-β and CUL5 E3 ligase complex by HIV-1 Vif
Structural basis for hijacking CBF-β and CUL5 E3 ligase complex by HIV-1 Vif
This study provides a crystal structure of the Vif–CBF-β–CUL5–ELOB–ELOC complex, which shows that Vif mimics the action of SOCS2 to interact with CUL5 and ELOC.
- Yingying Guo
- , Liyong Dong
- & Zhiwei Huang
-
Letter |
 Structure of a Naegleria Tet-like dioxygenase in complex with 5-methylcytosine DNA
Structure of a Naegleria Tet-like dioxygenase in complex with 5-methylcytosine DNA
The Tet family of dioxygenase enzymes convert 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine, which has an effect on gene expression; here the structure of NgTet1, a Tet-like protein with the same activity as mammalian Tet1, is determined, showing that NgTet1 uses a base-flipping mechanism to access 5-methylcytosine.
- Hideharu Hashimoto
- , June E. Pais
- & Xiaodong Cheng
-
Letter |
 Trapping the dynamic acyl carrier protein in fatty acid biosynthesis
Trapping the dynamic acyl carrier protein in fatty acid biosynthesis
A highly specific chemical crosslinking method is used to trap a complex between an acyl carrier protein and a fatty acid dehydratase during fatty acid biosynthesis; subsequent X-ray crystallography, NMR spectroscopy and molecular dynamics simulations techniques enable the detailed study of this complex.
- Chi Nguyen
- , Robert W. Haushalter
- & Michael D. Burkart
-
Article |
 Architecture of the large subunit of the mammalian mitochondrial ribosome
Architecture of the large subunit of the mammalian mitochondrial ribosome
Cryo-electron microscopy combined with chemical crosslinking and mass spectrometry is used to determine the structure of the large subunit of the mammalian mitoribosome; this structure provides detailed structural insight, particularly of the molecular architecture of the polypeptide exit site, which has been structurally remodelled during evolution, presumably to help facilitate the membrane insertion of the highly hydrophobic proteins encoded by the mitochondrial genome.
- Basil J. Greber
- , Daniel Boehringer
- & Nenad Ban

 Visualization of arrestin recruitment by a G-protein-coupled receptor
Visualization of arrestin recruitment by a G-protein-coupled receptor