Featured
-
-
Article
| Open Access Molecular mechanism for the synchronized electrostatic coacervation and co-aggregation of alpha-synuclein and tau
Molecular mechanism for the synchronized electrostatic coacervation and co-aggregation of alpha-synuclein and tau
Here, the authors report that α-synuclein phase-separates into liquid condensates with positively charged polypeptides such as Tau. The condensates undergo different maturation processes, including the formation of α-synuclein/Tau amyloid hetero-aggregates inside the condensates.
- Pablo Gracia
- , David Polanco
- & Nunilo Cremades
-
Article
| Open Access Disease-associated mutations within the yeast DNAJB6 homolog Sis1 slow conformer-specific substrate processing and can be corrected by the modulation of nucleotide exchange factors
Disease-associated mutations within the yeast DNAJB6 homolog Sis1 slow conformer-specific substrate processing and can be corrected by the modulation of nucleotide exchange factors
Here the authors describe mechanisms through which analogous LGMDD1 (Limb-Girdle Muscular Dystrophy Type D1) mutations affect Sis1 (a yeast functional homolog of human DNAJB6) chaperone activity and poison the function of wild-type protein; potentially uncovering a new therapeutic route to explore.
- Ankan K. Bhadra
- , Michael J. Rau
- & Heather L. True
-
Article
| Open Access The Cryo-EM structures of two amphibian antimicrobial cross-β amyloid fibrils
The Cryo-EM structures of two amphibian antimicrobial cross-β amyloid fibrils
In this work the authors provide high-resolution structural support for the amyloid-antimicrobial link via functional amyloids displaying propeller-like and kinked cross-β fibrils.
- Robert Bücker
- , Carolin Seuring
- & Meytal Landau
-
Article
| Open Access Heparin induces α-synuclein to form new fibril polymorphs with attenuated neuropathology
Heparin induces α-synuclein to form new fibril polymorphs with attenuated neuropathology
The Cryo-EM structures reported in this work reveal how heparin incorporates into α-syn fibril formation to determine fibril polymorphs. This highlights the role of biological polymers in the conformational selection and neuropathological regulation of amyloid fibrils.
- Youqi Tao
- , Yunpeng Sun
- & Dan Li
-
Article
| Open Access Modulating co-translational protein folding by rational design and ribosome engineering
Modulating co-translational protein folding by rational design and ribosome engineering
The narrow exit tunnel of the ribosome is important for cotranslational protein folding. Here, authors show that their rationally designed and engineered exit tunnel protein loops modulate the free energy of nascent chain dynamics and folding.
- Minkoo Ahn
- , Tomasz Włodarski
- & John Christodoulou
-
Article
| Open Access Trigger factor both holds and folds its client proteins
Trigger factor both holds and folds its client proteins
Using Guanidium HCl denatured glyceraldehyde 3-phosphate dehydrogenase (GAPDH), the authors provide in vitro evidence for the active involvement of the trigger factor (TF) in promoting the native folding of pre-unfolded client proteins, by preventing non-productive self-associations (misfolding) and by shielding oligomeric interfaces from aggregation.
- Kevin Wu
- , Thomas C. Minshull
- & James C. A. Bardwell
-
Comment
| Open Access The shape of things to come: structural insights into how prion proteins encipher heritable information
The shape of things to come: structural insights into how prion proteins encipher heritable information
The prion hypothesis embodies the radical concept that prion proteins contain the necessary information for infectious replication within their shape, thus obviating the requirement for genomic material. Two elegant papers by Hoyt et al. and Manka et al. describing high-resolution structures of infectious prions bring us closer to answering the long-standing question of how different prion conformations produce heritably distinct diseases.
- Glenn C. Telling
-
Article
| Open Access 2.7 Å cryo-EM structure of ex vivo RML prion fibrils
2.7 Å cryo-EM structure of ex vivo RML prion fibrils
High-resolution structures of mammalian prions have remained elusive. Here, Manka et al. report the cryo-EM structure of infectious RML prion fibrils from mice. Structural similarity with recently reported infectious 263K prion fibrils from hamsters now suggests a common prion architecture.
- Szymon W. Manka
- , Wenjuan Zhang
- & Jonathan D. F. Wadsworth
-
Article
| Open Access Many dissimilar NusG protein domains switch between α-helix and β-sheet folds
Many dissimilar NusG protein domains switch between α-helix and β-sheet folds
Folded proteins are composed of secondary structures, α-helices and β-sheets, that are generally assumed to be stable. Here, the authors combine computational prediction with experimental validation to show that many sequence-diverse NusG protein domains switch completely from α-helix to β-sheet folds.
- Lauren L. Porter
- , Allen K. Kim
- & Marie-Paule Strub
-
Article
| Open Access Structural basis for defective membrane targeting of mutant enzyme in human VLCAD deficiency
Structural basis for defective membrane targeting of mutant enzyme in human VLCAD deficiency
Prew et al. uncovered a structural basis for human VLCAD deficiency that arises from point mutations within the enzyme’s membrane-binding region, which was shown to fold as a putative α-helical hairpin. Helix-breaking mutations selectively disrupt membrane interaction and thus homeostatic function.
- Michelle S. Prew
- , Christina M. Camara
- & Loren D. Walensky
-
Article
| Open Access Coordination of metal center biogenesis in human cytochrome c oxidase
Coordination of metal center biogenesis in human cytochrome c oxidase
Mitochondrial cytochrome c oxidase is a heme aa3-copper oxygen reductase. Here, authors report that metal center-specific metallochaperones form dynamic assemblies to control heme a biosynthesis and coordinate copper transfer to the copper sites.
- Eva Nývltová
- , Jonathan V. Dietz
- & Antoni Barrientos
-
Article
| Open Access Cryo-EM structure of an amyloid fibril formed by full-length human SOD1 reveals its conformational conversion
Cryo-EM structure of an amyloid fibril formed by full-length human SOD1 reveals its conformational conversion
Misfolded SOD1 has been linked to both familial and sporadic ALS. Here the authors have determined the cryo-EM structure of SOD1 fibrils, providing insights into the conversion of SOD1 from its immature form into an aggregated form during pathogenesis of ALS.
- Li-Qiang Wang
- , Yeyang Ma
- & Yi Liang
-
Article
| Open Access Structural remodeling of ribosome associated Hsp40-Hsp70 chaperones during co-translational folding
Structural remodeling of ribosome associated Hsp40-Hsp70 chaperones during co-translational folding
Ribosome associated complex (RAC)- HSP70 (Ssb in yeast) is a eukaryotic chaperone system involved in co-translational folding. Here, authors report structures of RAC-containing ribosomal complexes, which suggest a working model for the dynamic actions of RAC-Ssb during the process.
- Yan Chen
- , Bin Tsai
- & Ning Gao
-
Article
| Open Access Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19
Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19
Here the authors report the formation of toxic clumps of protein, similar to amyloid assemblies found in Alzheimer’s disease and suggest their possible role for some of the neurological symptoms of long-COVID.
- Mirren Charnley
- , Saba Islam
- & Nicholas P. Reynolds
-
Article
| Open Access Universal protein misfolding intermediates can bypass the proteostasis network and remain soluble and less functional
Universal protein misfolding intermediates can bypass the proteostasis network and remain soluble and less functional
Nissley and co-workers predict protein misfolding into kinetically trapped, entangled conformations that can bypass the proteostasis network to remain soluble but less-functional for long timescales is widespread within the E. coli proteome.
- Daniel A. Nissley
- , Yang Jiang
- & Edward P. O’Brien
-
Article
| Open Access Fluent molecular mixing of Tau isoforms in Alzheimer’s disease neurofibrillary tangles
Fluent molecular mixing of Tau isoforms in Alzheimer’s disease neurofibrillary tangles
The tau protein in Alzheimer’s disease contains two isoforms. Using solid-state NMR and seeded growth of isotopically labeled tau, here the authors determined that the two isoforms mix fluently on the molecular level to propagate the AD tau structure.
- Aurelio J. Dregni
- , Pu Duan
- & Mei Hong
-
Article
| Open Access Improving recombinant protein production by yeast through genome-scale modeling using proteome constraints
Improving recombinant protein production by yeast through genome-scale modeling using proteome constraints
Due to the complexity of the protein secretory pathway, strategy suitable for the production of a certain recombination protein cannot be generalized. Here, the authors construct a proteome-constrained genome-scale protein secretory model for yeast and show its application in the production of different misfolded or recombinant proteins.
- Feiran Li
- , Yu Chen
- & Jens Nielsen
-
Article
| Open Access Insights into the client protein release mechanism of the ATP-independent chaperone Spy
Insights into the client protein release mechanism of the ATP-independent chaperone Spy
How ATP-independent chaperones release their clients without energy input remains enigmatic. Here the authors discover that chaperone Spy uses its long, disordered N terminus to facilitate client release through competitive, dynamic intramolecular interactions with Spy’s client binding surface.
- Wei He
- , Xinming Li
- & Shu Quan
-
Article
| Open Access Codon-specific Ramachandran plots show amino acid backbone conformation depends on identity of the translated codon
Codon-specific Ramachandran plots show amino acid backbone conformation depends on identity of the translated codon
Genetic code redundancies are considered inconsequential to protein structure. This study uncovers a dependence between local amino acid conformation in folded proteins and the identity of the codon from which that amino acid was translated.
- Aviv A. Rosenberg
- , Ailie Marx
- & Alex M. Bronstein
-
Article
| Open Access Templated folding of the RTX domain of the bacterial toxin adenylate cyclase revealed by single molecule force spectroscopy
Templated folding of the RTX domain of the bacterial toxin adenylate cyclase revealed by single molecule force spectroscopy
The authors use optical tweezers to show that the folding of repeats-in-toxin (RTX) block-iv in adenylate cyclase is templated by the folded RTX block-v. The findings suggest a possible mechanism for transmitting the folding signal in the RTX domain.
- Han Wang
- , Guojun Chen
- & Hongbin Li
-
Article
| Open Access Ca2+-mediated higher-order assembly of heterodimers in amino acid transport system b0,+ biogenesis and cystinuria
Ca2+-mediated higher-order assembly of heterodimers in amino acid transport system b0,+ biogenesis and cystinuria
Cystinuria is caused by mutations in heterodimeric amino acid transporter known as system b0,+. Here, authors discover that Ca2+ stabilizes the interface between two system b0,+ regulatory subunits rBAT, leading to super-dimerization of the b0,+AT–rBAT heterodimer, facilitating system b0,+ maturation.
- Yongchan Lee
- , Pattama Wiriyasermkul
- & Shushi Nagamori
-
Article
| Open Access Hyperphosphorylated tau self-assembles into amorphous aggregates eliciting TLR4-dependent responses
Hyperphosphorylated tau self-assembles into amorphous aggregates eliciting TLR4-dependent responses
In this work, the authors report that hyperphosphorylated recombinant tau spontaneously assembles into small, amorphous aggregates, which disrupt membranes and induce Toll-like receptor 4-dependent responses in human macrophages.
- Jonathan X. Meng
- , Yu Zhang
- & David Klenerman
-
Article
| Open Access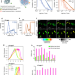 Stress-induced protein disaggregation in the endoplasmic reticulum catalysed by BiP
Stress-induced protein disaggregation in the endoplasmic reticulum catalysed by BiP
Aggregation of misfolded proteins underlie dementias. Here, the authors show that stressed cells activate an innate mechanism to resolve aggregates of defective proteins in the endoplasmic reticulum, where a third of cellular proteins are produced.
- Eduardo Pinho Melo
- , Tasuku Konno
- & Edward Avezov
-
Article
| Open Access Three-dimensional structure determination of protein complexes using matrix-landing mass spectrometry
Three-dimensional structure determination of protein complexes using matrix-landing mass spectrometry
Mass spectrometry (MS) is a powerful tool for the structural characterization of protein complexes. Here the authors offer a path for direct integration of MS and electron microscopy with a MS approach that enables grid deposition and structural preservation of gaseous protein complex ions.
- Michael S. Westphall
- , Kenneth W. Lee
- & Joshua J. Coon
-
Article
| Open Access Hidden information on protein function in censuses of proteome foldedness
Hidden information on protein function in censuses of proteome foldedness
Proteomics can define features of proteome foldedness by assessing the reactivity of surface exposed amino acids. Here, the authors show that such exposure patterns yield insight to structural changes in chaperones as they bind to unfolded proteins in urea-denatured mammalian cell lysate.
- Dezerae Cox
- , Ching-Seng Ang
- & Danny M. Hatters
-
Article
| Open Access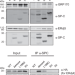 Mapping SP-C co-chaperone binding sites reveals molecular consequences of disease-causing mutations on protein maturation
Mapping SP-C co-chaperone binding sites reveals molecular consequences of disease-causing mutations on protein maturation
Interstitial Lung Disease (ILD)-associated mutations in surfactant protein C (SP-C) render the protein prone to aggregation. Here, the authors reveal their impact on protein maturation, provide insights into recognition of aggregation prone regions by chaperones, and address the autosomal dominant nature of ILD mutants.
- Kristine F. R. Pobre-Piza
- , Melissa J. Mann
- & Linda M. Hendershot
-
Article
| Open Access Structural basis of FPR2 in recognition of Aβ42 and neuroprotection by humanin
Structural basis of FPR2 in recognition of Aβ42 and neuroprotection by humanin
The formyl peptide receptor 2 (FPR2) is involved in the pathogenesis of Alzheimer’s disease. Structures of FPR2 bound to Aβ42, humanin, or formyl peptides offer insight into Aβ42 neurotoxicity, humanin neuroprotection, and FPR ligand selectivity
- Ya Zhu
- , Xiaowen Lin
- & Beili Wu
-
Article
| Open Access Cyclophilin anaCyp40 regulates photosystem assembly and phycobilisome association in a cyanobacterium
Cyclophilin anaCyp40 regulates photosystem assembly and phycobilisome association in a cyanobacterium
Cyclophilins are proteins found in many organisms, where they can play roles as chaperones, in signal transduction, or other functions. Here, Yadav et al. show that a cyanobacterial cyclophilin is involved in stress responses and in assembly of photosynthetic complexes, and displays unique structural features.
- Shivam Yadav
- , Martin Centola
- & Enrico Schleiff
-
Article
| Open Access Characterization of protein unfolding by fast cross-linking mass spectrometry using di-ortho-phthalaldehyde cross-linkers
Characterization of protein unfolding by fast cross-linking mass spectrometry using di-ortho-phthalaldehyde cross-linkers
Conformations sampled by a protein while it unfolds are difficult to visualize. Here, the authors develop di-ortho-phthalaldehyde cross-linkers for rapid chemical cross-linking mass spectrometry analysis and demonstrate that this method captures the conformations of protein unfolding intermediates.
- Jian-Hua Wang
- , Yu-Liang Tang
- & Xiaoguang Lei
-
Article
| Open Access Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers
Mapping the sequence specificity of heterotypic amyloid interactions enables the identification of aggregation modifiers
In this work, Louros et al. uncover a rule book for interactions of amyloids with other proteins. This grammar was shown to promote cellular spreading of tau aggregates in cells, but can also be harvested to develop structure-based aggregation blockers.
- Nikolaos Louros
- , Meine Ramakers
- & Joost Schymkowitz
-
Article
| Open Access Spatiotemporal modulations in heterotypic condensates of prion and α-synuclein control phase transitions and amyloid conversion
Spatiotemporal modulations in heterotypic condensates of prion and α-synuclein control phase transitions and amyloid conversion
The authors show that prion protein and α-synuclein undergo phase separation through domain-specific electrostatic interactions. These complex coacervates possess electrostatic nanoclusters and can convert into multiphasic condensates and amyloids.
- Aishwarya Agarwal
- , Lisha Arora
- & Samrat Mukhopadhyay
-
Article
| Open Access Differential roles for DNAJ isoforms in HTT-polyQ and FUS aggregation modulation revealed by chaperone screens
Differential roles for DNAJ isoforms in HTT-polyQ and FUS aggregation modulation revealed by chaperone screens
Here, using quantitative screens in human cells, the authors reveal distinct effects of naturally-occurring DNAJ chaperone isoforms on pathological aggregation of the Huntington’s disease-associated HTT-polyQ and the ALS-related mutant FUS.
- Kinneret Rozales
- , Amal Younis
- & Reut Shalgi
-
Article
| Open Access Towards a generic prototyping approach for therapeutically-relevant peptides and proteins in a cell-free translation system
Towards a generic prototyping approach for therapeutically-relevant peptides and proteins in a cell-free translation system
Generic approach for rapid prototyping is essential for the progress of synthetic biology. Here the authors modify the cell-free translation system to control protein aggregation and folding and validate the approach by using single conditions for prototyping of various disulfide-constrained polypeptides.
- Yue Wu
- , Zhenling Cui
- & Sergey Mureev
-
Article
| Open Access Cryo-EM demonstrates the in vitro proliferation of an ex vivo amyloid fibril morphology by seeding
Cryo-EM demonstrates the in vitro proliferation of an ex vivo amyloid fibril morphology by seeding
Here, the authors present the cryo-EM structure of in vitro amyloid fibrils from recombinant SAA1.1 protein that were formed by seeding with fibrils purified from systemic AA amyloidosis tissue. This in vitro fibril structure resembles the structure of the ex vivo fibrils but differs from unseeded in vitro fibrils. These findings show that fibril morphologies can be propagated in vitro by seeding.
- Thomas Heerde
- , Matthies Rennegarbe
- & Marcus Fändrich
-
Article
| Open Access Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α
Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α
IRE1 helps mitigate endoplasmic-reticulum stress by cleaving specific mRNAs at a conserved sequence endomotif via regulated IRE1-dependent decay (RIDD). Here the authors discover a more promiscuous IRE1 activity dubbed RIDD lacking endomotif (RIDDLE).
- Adrien Le Thomas
- , Elena Ferri
- & Avi Ashkenazi
-
Article
| Open Access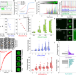 Capillary flow experiments for thermodynamic and kinetic characterization of protein liquid-liquid phase separation
Capillary flow experiments for thermodynamic and kinetic characterization of protein liquid-liquid phase separation
Methods to quantitatively study liquid-liquid phase separation (LLPS) of proteins are lacking. Here the authors report Capillary flow experiments (Capflex) for the quantification of key LLPS parameters; they study Ddx4, the RP3 peptide and the aberrant liquid-to-solid phase transition of α-synuclein.
- Emil G. P. Stender
- , Soumik Ray
- & Alexander K. Buell
-
Article
| Open Access Plasticity within the barrel domain of BamA mediates a hybrid-barrel mechanism by BAM
Plasticity within the barrel domain of BamA mediates a hybrid-barrel mechanism by BAM
The β-barrel assembly machinery (BAM) assists the folding and membrane insertion of bacterial outer membrane proteins. Here, the authors report structural characterization of BAM in lipid environment and in complex with the client protein EspP integrated into the barrel of BamA, providing insight into BAM mechanism of function.
- Runrun Wu
- , Jeremy W. Bakelar
- & Nicholas Noinaj
-
Article
| Open Access Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis
Plasmin activity promotes amyloid deposition in a transgenic model of human transthyretin amyloidosis
ATTR amyloidosis causes heart failure through the accumulation of misfolded transthyretin in cardiac muscle. Here the authors report a mouse model of ATTR amyloidosis and demonstrate the involvement of protease activity in ATTR amyloid deposition.
- Ivana Slamova
- , Rozita Adib
- & J. Paul Simons
-
Article
| Open Access Monitoring the binding and insertion of a single transmembrane protein by an insertase
Monitoring the binding and insertion of a single transmembrane protein by an insertase
The insertion and folding nascent or fully synthesized polypeptides into membranes is assisted by insertases. Here, the authors use a range of biophysical approaches to provide molecular details of how the transmembrane insertase YidC facilitates the insertion a protein into a phospholipid membrane.
- Pawel R. Laskowski
- , Kristyna Pluhackova
- & Daniel J. Müller
-
Article
| Open Access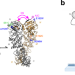 Two-colour single-molecule photoinduced electron transfer fluorescence imaging microscopy of chaperone dynamics
Two-colour single-molecule photoinduced electron transfer fluorescence imaging microscopy of chaperone dynamics
Revealing mechanisms of complex protein machines requires simultaneous exploration of multiple structural coordinates. Here the authors report two-colour fluorescence microscopy combined with photoinduced electron transfer probes to simultaneously detect two structural coordinates in single protein molecules.
- Jonathan Schubert
- , Andrea Schulze
- & Hannes Neuweiler
-
Article
| Open Access Plant LHC-like proteins show robust folding and static non-photochemical quenching
Plant LHC-like proteins show robust folding and static non-photochemical quenching
Plant light harvesting complex (LHC)‐like proteins protect the photosynthetic machinery from excess light. Here the authors show that plant LHC‐like dimers are stabilized by associated pigments and can quench chlorophyll fluorescence via direct energy transfer from chlorophyll to zeaxanthin.
- Petra Skotnicová
- , Hristina Staleva-Musto
- & Roman Sobotka
-
Article
| Open Access Phosphorylation activates the yeast small heat shock protein Hsp26 by weakening domain contacts in the oligomer ensemble
Phosphorylation activates the yeast small heat shock protein Hsp26 by weakening domain contacts in the oligomer ensemble
Small heat shock proteins (sHsps) form large spherical assemblies and their regulation is not well understood. Here, the authors provide insights into the mechanism of Hsp26 activation by characterising phospho-mimetic mutants of yeast Hsp26. They present cryo-EM structures of the wild-type Hsp26 40mer and its phospho-mimetic mutants that reveal the location of the thermosensor in the oligomer, and the authors also show that the thermosensor domain is targeted by phosphorylation, which relieves the intrinsic inhibition of chaperone activity.
- Moritz Mühlhofer
- , Carsten Peters
- & Johannes Buchner
-
Article
| Open Access Nuclear and cytoplasmic huntingtin inclusions exhibit distinct biochemical composition, interactome and ultrastructural properties
Nuclear and cytoplasmic huntingtin inclusions exhibit distinct biochemical composition, interactome and ultrastructural properties
The mechanisms underlying Huntingtin protein (Htt) aggregation are not fully understood. Here the authors perform a detailed investigation of the ultrastructural and biochemical properties of huntingtin cytoplasmic and nuclear inclusions, and reveal that they form via distinct mechanisms and exert their toxicity via different pathways.
- Nathan Riguet
- , Anne-Laure Mahul-Mellier
- & Hilal A. Lashuel
-
Article
| Open Access Dissection of the amyloid formation pathway in AL amyloidosis
Dissection of the amyloid formation pathway in AL amyloidosis
AL amyloidosis is caused by the accumulation of overproduced light chain (LC) fragments as fibrils in patient organs and it is the most prevalent systemic amyloidosis. Here, the authors combine biochemical and biophysical experiments to characterise the lag phase of a patient-derived truncated LC and they identify structural transitions that precede fibril formation.
- Pamina Kazman
- , Ramona M. Absmeier
- & Johannes Buchner
-
Article
| Open Access Nascent chains can form co-translational folding intermediates that promote post-translational folding outcomes in a disease-causing protein
Nascent chains can form co-translational folding intermediates that promote post-translational folding outcomes in a disease-causing protein
Alpha-1-antitrypsin (AAT) deficiency results from misfolding-prone AAT variants. Here the authors show that AAT forms co-translational folding intermediates on the ribosome that persist upon release and determine its folding fate. They show too that the ribosome can also modulate misfolding-prone AAT intermediates during their synthesis.
- Elena Plessa
- , Lien P. Chu
- & Lisa D. Cabrita
-
Article
| Open Access Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM
Role of mutations and post-translational modifications in systemic AL amyloidosis studied by cryo-EM
Systemic AL amyloidosis is caused by misfolding of immunoglobulin light chains (LCs) but how post-translational modifications (PTMs) of LCs influence amyloid formation is not well understood. Here, the authors present the cryo-EM structure of an AL amyloid fibril derived from the heart tissue of a patient that is partially pyroglutamylated, N-glycosylated and contains an intramolecular disulfide bond. Based on their structure and biochemical experiments the authors conclude that the mutational changes, disulfide bond and glycosylation determine the fibril protein fold and that glycosylation protects the fibril core from proteolytic degradation.
- Lynn Radamaker
- , Sara Karimi-Farsijani
- & Marcus Fändrich
-
Article
| Open Access Dynamic interactions and Ca2+-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding
Dynamic interactions and Ca2+-binding modulate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding
The calcium binding protein S100B is an abundantly expressed protein in the brain and has neuro-protective functions by inhibiting Aβ aggregation and metal ion toxicity. Here, the authors combine cell biology and biochemical experiments with chemical kinetics and NMR measurements and show that S100B protein is an extracellular Tau chaperone and further characterize the interactions between S100B and Tau.
- Guilherme G. Moreira
- , François-Xavier Cantrelle
- & Cláudio M. Gomes
-
Article
| Open Access A dual-reporter system for investigating and optimizing protein translation and folding in E. coli
A dual-reporter system for investigating and optimizing protein translation and folding in E. coli
Heterologous expression of recombinant proteins often results in misfolding, aggregation and degradation. Here, we show an in vivo dual-biosensor system that simultaneously assesses protein translation and protein folding, thereby enabling rapid screening of expression strains as well as mutant libraries.
- Ariane Zutz
- , Louise Hamborg
- & Alex Toftgaard Nielsen
-
Article
| Open Access The Hsc70 disaggregation machinery removes monomer units directly from α-synuclein fibril ends
The Hsc70 disaggregation machinery removes monomer units directly from α-synuclein fibril ends
Molecular chaperones from the Hsp70 family can break up protein aggregates, including amyloids. Here, the authors utilize microfluidic diffusional sizing to assess the mechanism of α-synuclein (αS) disaggregation by the Hsc70–DnaJB1–Apg2 system, and show that single αS molecules are removed directly from the fibril ends.
- Matthias M. Schneider
- , Saurabh Gautam
- & Tuomas P. J. Knowles

 Identification of a HTT-specific binding motif in DNAJB1 essential for suppression and disaggregation of HTT
Identification of a HTT-specific binding motif in DNAJB1 essential for suppression and disaggregation of HTT