Article
|
Open Access
Featured
-
-
Article
| Open Access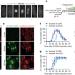 Core control principles of the eukaryotic cell cycle
Core control principles of the eukaryotic cell cycle
The core cell cycle is largely driven by increasing total CDK activity together with minor differences in the substrate specificity of the CDKs initiating DNA replication and mitosis.
- Souradeep Basu
- , Jessica Greenwood
- & Paul Nurse
-
Article |
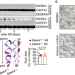 MARK4 controls ischaemic heart failure through microtubule detyrosination
MARK4 controls ischaemic heart failure through microtubule detyrosination
MARK4 regulates cardiomyocyte contractility by promoting MAP4 phosphorylation, which facilitates the access of VASH2 to microtubules for the detyrosination of α-tubulin; MARK4 deficiency after acute myocardial infarction limits the reduction in the left ventricular ejection fraction.
- Xian Yu
- , Xiao Chen
- & Xuan Li
-
Article |
 Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms
Splicing factor YBX1 mediates persistence of JAK2-mutated neoplasms
Inhibition of YBX1, a downstream target of the Janus kinase JAK2, sensitizes myeloproliferative neoplasm cells to JAK and could provide a means to eradicate such cells in human haematopoietic cancers.
- Ashok Kumar Jayavelu
- , Tina M. Schnöder
- & Florian H. Heidel
-
Article |
 Regulation of α-synuclein by chaperones in mammalian cells
Regulation of α-synuclein by chaperones in mammalian cells
Chaperones interact with a canonical motif in α-synuclein, which can be prevented by phosphorylation of α-synuclein at Tyr39, whereas inhibition of this interaction leads to the localization of α-synuclein to the mitochondria and aggregate formation.
- Björn M. Burmann
- , Juan A. Gerez
- & Sebastian Hiller
-
Article |
 Dynamic lineage priming is driven via direct enhancer regulation by ERK
Dynamic lineage priming is driven via direct enhancer regulation by ERK
ERK reversibly regulates embryonic stem cell transcription via selective redistribution of co-factors and RNA polymerase from pluripotency to early differentiation enhancers, while leaving transcription factors bound to their enhancers, thus preserving plasticity.
- William B. Hamilton
- , Yaron Mosesson
- & Joshua M. Brickman
-
Letter |
 p38γ is essential for cell cycle progression and liver tumorigenesis
p38γ is essential for cell cycle progression and liver tumorigenesis
The stress-activated kinase p38γ has a role in regulating entry into the cell cycle; in the liver, it can induce cellular proliferation during regeneration and promote the development of hepatocellular carcinoma.
- Antonia Tomás-Loba
- , Elisa Manieri
- & Guadalupe Sabio
-
Letter |
 PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress
PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress
Phosphorylation of one of two adjacent serine residues in TSC2 is both required and sufficient for PKG1-mediated cardiac protection against pressure overload in mice; these serine residues provide a genetic tool for the bidirectional regulation of stress-stimulated mTORC1 activity.
- Mark J. Ranek
- , Kristen M. Kokkonen-Simon
- & David A. Kass
-
Article |
 Kinase-controlled phase transition of membraneless organelles in mitosis
Kinase-controlled phase transition of membraneless organelles in mitosis
The dual-specificity kinase DYRK3 acts as a central ‘dissolvase’, mediating the phase transitions of several types of membraneless organelles during mitosis.
- Arpan Kumar Rai
- , Jia-Xuan Chen
- & Lucas Pelkmans
-
Letter |
 The protein histidine phosphatase LHPP is a tumour suppressor
The protein histidine phosphatase LHPP is a tumour suppressor
Decreased expression of histidine phosphatase LHPP, a novel tumour suppressor, results in increased global histidine phosphorylation and hepatocellular carcinoma.
- Sravanth K. Hindupur
- , Marco Colombi
- & Michael N. Hall
-
Article |
 Structure of PINK1 in complex with its substrate ubiquitin
Structure of PINK1 in complex with its substrate ubiquitin
Stabilization of a transient protein kinase–substrate complex using a nanobody provides molecular details about how the Parkinson’s disease-linked protein kinase PINK1 phosphorylates ubiquitin, and suggests new pharmacological strategies.
- Alexander F. Schubert
- , Christina Gladkova
- & David Komander
-
Article |
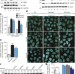 The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy
The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy
The PINK1 ubiquitin kinase is shown to recruit the two autophagy receptors NDP52 and OPTN to mitochondria to activate mitophagy directly, independently of the ubiquitin ligase parkin; once recruited to mitochondria, NDP52 and OPTN recruit autophagy initiation components, and parkin may amplify the phospho-ubiquitin signal generated by PINK1, resulting in robust autophagy induction.
- Michael Lazarou
- , Danielle A. Sliter
- & Richard J. Youle
-
Letter |
 Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus
Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus
Phosphorylation of Akt at its carboxy-terminal tail is an essential layer of Akt activation to regulate its physiological functions.
- Pengda Liu
- , Michael Begley
- & Wenyi Wei
-
Article |
 Temporal regulation of EGF signalling networks by the scaffold protein Shc1
Temporal regulation of EGF signalling networks by the scaffold protein Shc1
The Shc1 scaffold mediates a switch in the signaling output of the epidermal growth factor receptor tyrosine kinase over time through recruitment of successive waves of proteins with distinct biological functions.
- Yong Zheng
- , Cunjie Zhang
- & Tony Pawson
-
News & Views |
 A division duet
A division duet
The orchestration of cell division requires a programme of events choreographed by protein modification. A study shows that the relative activity of a phosphatase enzyme towards its substrates imposes order during the final act of division.
- Curt Wittenberg
-
Letter |
 A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK
A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK
- Damian F. Brennan
- , Arvin C. Dar
- & David Barford
-
Letter |
 Damage-induced phosphorylation of Sld3 is important to block late origin firing
Damage-induced phosphorylation of Sld3 is important to block late origin firing
Two classes of enzyme — cyclin-dependent kinases (CDK) and Dbf4-dependent kinase (DDK) — facilitate the initiation of DNA replication in eukaryotes. It is now shown that, when DNA damage is sensed, another kinase, Rad53, halts the firing of late replication origins by inhibiting both the CDK and the DDK pathways. Rad53 acts on DDK directly by inhibiting Dbf4, whereas the CDK pathway is blocked by Rad53-mediated phosphorylation of the downstream CDK substrate Sld3.
- Jaime Lopez-Mosqueda
- , Nancy L. Maas
- & David P. Toczyski
-
Letter |
 Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation
Checkpoint-dependent inhibition of DNA replication initiation by Sld3 and Dbf4 phosphorylation
Two classes of enzyme — cyclin-dependent kinases (CDK) and Dbf4-dependent kinase (DDK) — facilitate the initiation of DNA replication in eukaryotes. It is now shown that, when DNA damage is sensed, another kinase, Rad53, halts the firing of late replication origins by inhibiting both the CDK and the DDK pathways. Rad53 acts on DDK directly by inhibiting Dbf4, whereas the CDK pathway is blocked by Rad53-mediated phosphorylation of the downstream CDK substrate Sld3.
- Philip Zegerman
- & John F. X. Diffley
-
Letter |
 Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation
Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation
The chromosomal passenger complex (CPC) coordinates several processes during cell division, including chromosome bi-orientation and cytokinesis, and its proper localization is crucial. These authors provide a mechanism for its localization to the inner centromere. Cdk1–cyclin-B-dependent phosphorylation of the CPC promotes binding to shugoshin, which the authors define as a conserved centromeric adaptor of the CPC. This mechanism is conserved between fission yeast and human cells and highlights a crucial role of Cdk1–cyclin B in chromosome bi-orientation.
- Tatsuya Tsukahara
- , Yuji Tanno
- & Yoshinori Watanabe
-
Letter |
 eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation
eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation
The initiation of protein synthesis requires the eukaryotic translation initiation factor (eIF) 2, which uses energy from the hydrolysis of GTP. Another factor, eIF5, accelerates the GTP-hydrolysing activity of eIF2. Here, two other roles for eIF5 have been defined. One involves stabilizing GDP, the product of GTP hydrolysis, on eIF2. In its other role, eIF5 works with phosphorylated eIF2 to inhibit the guanine-nucleotide exchange factor eIF2B. These results clarify our understanding of how the initiation of translation is regulated.
- Martin D. Jennings
- & Graham D. Pavitt

 An atlas of substrate specificities for the human serine/threonine kinome
An atlas of substrate specificities for the human serine/threonine kinome