Featured
-
-
Article
| Open Access Phosphoric acid-catalyzed atroposelective construction of axially chiral arylpyrroles
Phosphoric acid-catalyzed atroposelective construction of axially chiral arylpyrroles
Axially chiral arylpyrroles are structural motifs often found in natural and medicinal products. Here, the authors report the atroposelective synthesis of axially chiral arylpyrrole derivatives, which proved to be efficient chiral ligands for asymmetric catalysis, through desymmetrization and kinetic resolution.
- Lei Zhang
- , Shao-Hua Xiang
- & Bin Tan
-
Article
| Open Access Catalytic enantioselective construction of axial chirality in 1,3-disubstituted allenes
Catalytic enantioselective construction of axial chirality in 1,3-disubstituted allenes
Highly enantioselective synthesis of allenes has been relying, so far, on the steric hindrance of substrates. Here the authors achieve excellent stereocontrol in the synthesis of chiral allenes with a palladium-DTBM-SEGPHOS catalytic system in a non-substrate-dependent manner.
- Shihua Song
- , Jing Zhou
- & Shengming Ma
-
Article
| Open Access Amide nitrogen pyramidalization changes lactam amide spinning
Amide nitrogen pyramidalization changes lactam amide spinning
Cis-trans lactam amide rotation is a fundamental process and its understanding might aid molecular design. Here, the authors report the synthesis and study of bicyclic lactams which undergo spin through 360 degrees as in open-chain amides, due to the occurrence of nitrogen pyramidalization.
- Yuko Otani
- , Xin Liu
- & Tomohiko Ohwada
-
Article
| Open Access Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications
Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications
Since fluoro-compounds are popular in industrial settings, efficient methods for incorporation of fluoride into organic molecules are desirable. Here, the authors developed trifluoroacetaldehyde N-tfsylhydrazone (TFHZ-Tfs) as a CF3CHN2 surrogate that generates CF3CHN2 in situ under basic conditions.
- Xinyu Zhang
- , Zhaohong Liu
- & Xihe Bi
-
Article
| Open Access A diversity-oriented rhodamine library for wide-spectrum bactericidal agents with low inducible resistance against resistant pathogens
A diversity-oriented rhodamine library for wide-spectrum bactericidal agents with low inducible resistance against resistant pathogens
Preparation of xanthene-containing compounds has been limited due to structural bias existing methods pose. Here, the authors developed a mild, diversity-oriented method for rhodamines synthesis, leading to the finding of compounds with antibacterial potency against a variety of bacterial species.
- Xiao Luo
- , Liujia Qian
- & Youjun Yang
-
Article
| Open Access Iron-catalyzed carboazidation of alkenes and alkynes
Iron-catalyzed carboazidation of alkenes and alkynes
Carboazidation of alkenes and alkynes holds the promise to construct valuable, functionalized molecules. Here, the authors report a general and efficient iron-catalyzed carboazidation of both alkenes and alkynes enabled by t-butyl perbenzoate.
- Haigen Xiong
- , Nagarajan Ramkumar
- & Hongli Bao
-
Article
| Open Access The synthesis and characterization of giant Calixarenes
The synthesis and characterization of giant Calixarenes
Calixarenes are cyclic oligomers obtained by condensation reactions between phenols and formaldehyde and usually include up to eight phenolic subunits. Here the authors demonstrate the synthesis of calixarenes with up to 90 phenolic subunits with up to 65% yield via a scalable process.
- Vincent Guérineau
- , Marion Rollet
- & Vincent Huc
-
Article
| Open Access Desymmetrization of meso-bisphosphates using copper catalysis and alkylzirconocene nucleophiles
Desymmetrization of meso-bisphosphates using copper catalysis and alkylzirconocene nucleophiles
Copper-catalyzed allylic desymmetrizations are, so far, limited to carbocyclic products with two tertiary stereocenters. Here, the authors report a highly enantioselective Cu-catalyzed desymmetrization of meso carbo- and hetero-cyclic bisphosphates to give products with up to three contiguous stereocenters.
- Reece Jacques
- , Robert D. C. Pullin
- & Stephen P. Fletcher
-
Article
| Open Access Click chemistry enables quantitative chiroptical sensing of chiral compounds in protic media and complex mixtures
Click chemistry enables quantitative chiroptical sensing of chiral compounds in protic media and complex mixtures
Chiroptical sensing in complex mixtures remains a challenging task. Here, the authors report an efficient coumarin probe for chiroptical click chirality sensing of absolute configuration, concentration and enantiomeric excess of several compound classes. The method can be directly applied to crude asymmetric reaction mixtures.
- F. Yushra Thanzeel
- , Kaluvu Balaraman
- & Christian Wolf
-
Article
| Open Access Irradiation-induced palladium-catalyzed decarboxylative desaturation enabled by a dual ligand system
Irradiation-induced palladium-catalyzed decarboxylative desaturation enabled by a dual ligand system
Production of alkenes through decarboxyolefination of carboxylates is a synthetically useful process. Here, the authors report a palladium/dual ligand-enabled decarboxylative desaturation reaction under mild irradiation conditions and apply it to the synthesis of Chondriamide A and C.
- Wan-Min Cheng
- , Rui Shang
- & Yao Fu
-
Article
| Open Access Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO
Differentiation between enamines and tautomerizable imines in the oxidation reaction with TEMPO
Tautomerization of imines into enamines is the basis of their similar reactivity; however, minor structural changes may lead to different outcomes. Here, the authors show that the reaction of cyclohexanone and amines in presence of TEMPO affords either α-amino-enones or arylamines depending on the intermediate imine structure.
- Xiaoming Jie
- , Yaping Shang
- & Weiping Su
-
Article
| Open Access Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds
Multidirectional desymmetrization of pluripotent building block en route to diastereoselective synthesis of complex nature-inspired scaffolds
Methods enabling the synthesis of diverse collections of nature-inspired compounds with potential medicinal use are sought after in drug design. Here, the authors report a build/couple/pair strategy to efficiently construct chiral polycyclic scaffolds and show their diversification for drug discovery screening.
- Vunnam Srinivasulu
- , Paul Schilf
- & Taleb H. Al-Tel
-
Article
| Open Access Cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides
Cobalt-catalyzed difluoroalkylation of tertiary aryl ketones for facile synthesis of quaternary alkyl difluorides
The catalytic C(sp3)-CF2 bond formation is of high interest in drug design and discovery. Here, the authors report a cobalt-catalyzed difluoroalkylation of tertiary α-C-H bonds of aryl ketones for facile synthesis of quaternary alkyl difluorides and show the late-stage functionalization of marketed drugs.
- Chao Li
- , Yi-Xuan Cao
- & Xi-Sheng Wang
-
Article
| Open Access Visible light-promoted CO2 fixation with imines to synthesize diaryl α-amino acids
Visible light-promoted CO2 fixation with imines to synthesize diaryl α-amino acids
Fixation of CO2 in organic molecules is an area of great interest due to the implications in sustainable chemistry. Here, the authors show a visible light-mediated hydrocarboxylation of ketimines with atmospheric CO2 to afford a number of α,α–diaryl α-amino acid derivatives.
- Xinyuan Fan
- , Xu Gong
- & Patrick J. Walsh
-
Article
| Open Access A third generation of radical fluorinating agents based on N-fluoro-N-arylsulfonamides
A third generation of radical fluorinating agents based on N-fluoro-N-arylsulfonamides
Common radical fluorination reagents, such as Selectofluor®, are penalized by the need for metal catalysts and possible oxidation side reactions. Here, the authors reported the synthesis and application of N-fluoro-N-arylsulfonamides (NFASs) as third generation of radical fluorinating reagents to overcome those limitations.
- Daniel Meyer
- , Harish Jangra
- & Philippe Renaud
-
Article
| Open Access Samarium(II) folding cascades involving hydrogen atom transfer for the synthesis of complex polycycles
Samarium(II) folding cascades involving hydrogen atom transfer for the synthesis of complex polycycles
Diversity-oriented synthesis is a valuable strategy to construct complex molecules of medicinal interest. Here, the authors show a folding cascade strategy to convert linear substrates into polycyclic compounds with multiple stereocentres by combining the reductive chemistry of SmI2 with 1,5-hydrogen atom transfer.
- Mateusz P. Plesniak
- , Monserrat H. Garduño-Castro
- & David J. Procter
-
Article
| Open Access N2H4 as traceless mediator for homo- and cross- aryl coupling
N2H4 as traceless mediator for homo- and cross- aryl coupling
Synthesis of biaryls by coupling strategies is unavoidably accompanied by stoichiometric metal waste. Here, the authors report the homo- and cross-electrophile coupling by using hydrazine as metal surrogate, only liberating N2 and H2 as side products.
- Leiyang Lv
- , Zihang Qiu
- & Chao-Jun Li
-
Article
| Open Access Design and 22-step synthesis of highly potent D-ring modified and linker-equipped analogs of spongistatin 1
Design and 22-step synthesis of highly potent D-ring modified and linker-equipped analogs of spongistatin 1
Step-economical and efficient syntheses of Spongistatin 1 analogs are desirable for the development of potent anti-proliferative agents. Here, the authors report a 22-step synthesis of a D-ring modified Spongistatin 1 analog with retained picomolar potency among a group of C(15) ester derivatives.
- Linda M. Suen
- , Makeda A. Tekle-Smith
- & James L. Leighton
-
Article
| Open Access A Chiron approach towards the stereoselective synthesis of polyfluorinated carbohydrates
A Chiron approach towards the stereoselective synthesis of polyfluorinated carbohydrates
Polyfluorinated hexopyranoses display unique physical, chemical and biological properties, however their stereoselective synthesis is highly challenging. Here, the authors report a synthetic approach based on the chemical manipulation of inexpensive levoglucosan to obtain heavily fluorinated monosaccharides stereoselectively.
- Vincent Denavit
- , Danny Lainé
- & Denis Giguère
-
Article
| Open Access syn-Selective alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis
syn-Selective alkylarylation of terminal alkynes via the combination of photoredox and nickel catalysis
Converting alkynes into alkenes with high stereoselectivity via two consecutive C-C bond forming steps is a desirable process, yet very challenging. Here, the authors describe a dual photoredox-nickel catalytic system for the regio- and syn-selective alkylarylation of terminal alkynes with alkyl oxalates and aryl bromides.
- Lei Guo
- , Fan Song
- & Lingling Chu
-
Article
| Open Access Multifunctional sequence-defined macromolecules for chemical data storage
Multifunctional sequence-defined macromolecules for chemical data storage
Sequence-defined macromolecules consist of a defined chain length and topology and can be used in applications such as antibiotics and data storage. Here the authors developed two algorithms to encode text fragments and QR codes as a collection of oligomers and to reconstruct the original data.
- Steven Martens
- , Annelies Landuyt
- & Filip Du Prez
-
Article
| Open Access Defluorosilylation of fluoroarenes and fluoroalkanes
Defluorosilylation of fluoroarenes and fluoroalkanes
Transformation of strong C-F bonds into C-Si bonds is an extremely useful strategy for further derivatization of organic molecules. Here, the authors report a nickel-catalyzed strategy to convert aryl fluorides into sylylated arenes while defluorosilylation of alkyl fluorides is achieved under metal-free conditions.
- Benqiang Cui
- , Shichong Jia
- & Norio Shibata
-
Article
| Open Access A selenium-catalysed para-amination of phenols
A selenium-catalysed para-amination of phenols
Selenium has emerged as a metalloid for the catalytic construction of C–N bonds; however no functionalisation of aromatic compounds has been developed yet. Here, the authors report the para-amination of phenols via two successive sigmatropic rearrangements of a redox versatile Se–N bond.
- Dingyuan Yan
- , Guoqiang Wang
- & Jing Zhao
-
Article
| Open Access Highly selective transition-metal-free transamidation of amides and amidation of esters at room temperature
Highly selective transition-metal-free transamidation of amides and amidation of esters at room temperature
Transamidation reactions usually require transition metal catalysts and/or harsh conditions. Here, the authors show a general and operationally-simple protocol for the transamidation of amides and amidation of esters by highly selective acyl cleavage with non-nucleophilic amines under mild conditions.
- Guangchen Li
- & Michal Szostak
-
Article
| Open Access Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy
Unravelling the structure of glycosyl cations via cold-ion infrared spectroscopy
Glycosyl cations are key intermediates in glycosylation reactions, but their structure has remained elusive due to their transient nature. Here, the authors perform an in-depth structural analysis and report that C2-participating protective groups induce acetoxonium cations with distinct ring conformations.
- Eike Mucha
- , Mateusz Marianski
- & Kevin Pagel
-
Article
| Open Access Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines
Simple ruthenium-catalyzed reductive amination enables the synthesis of a broad range of primary amines
Synthesis of primary amines via operationally simple, inexpensive and environmentally friendly methodologies has high impact in industrial settings. Here, the authors show a reductive amination process involving a ruthenium catalyst, aldehydes/ketones, ammonia, and hydrogen that displays a remarkable scope of primary amine products.
- Thirusangumurugan Senthamarai
- , Kathiravan Murugesan
- & Rajenahally V. Jagadeesh
-
Article
| Open Access An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy
An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy
Non-covalent glycosyl donor activation often requires high organocatalyst loadings. Here, the authors demonstrate that strain-release glycosylations can take place at very low thiourea catalyst loadings. In addition, the authors developed a one-pot multicatalytic strategy that can diversify glycosides rapidly.
- Chunfa Xu
- & Charles C. J. Loh
-
Article
| Open Access Total syntheses of shizukaols A and E
Total syntheses of shizukaols A and E
Shizukaols, bioactive dimers naturally occurring in the Chloranthaceae family, possess a complex polycyclic framework with more than ten contiguous stereocenters. Here the authors report the total syntheses of shizukaols A and E achieved via a modified Diels–Alder reaction mimicking the biosynthetic pathway.
- Jian-Li Wu
- , Yin-Suo Lu
- & Xiao-Shui Peng
-
Article
| Open Access Dye-incorporated coordination polymers for direct photocatalytic trifluoromethylation of aromatics at metabolically susceptible positions
Dye-incorporated coordination polymers for direct photocatalytic trifluoromethylation of aromatics at metabolically susceptible positions
Direct trifluoromethylation of unactivated aromatic rings at metabolically susceptible positions is highly desirable in pharmaceutical fields. Here, the authors report a new approach for direct trifluoromethylation of unactivated aromatics by incorporating thiophenes into the backbone of triphenylamine-based ligand.
- Tiexin Zhang
- , Xiangyang Guo
- & Chunying Duan
-
Article
| Open Access Superplasticity in an organic crystal
Superplasticity in an organic crystal
Superplasticity enables processing on hard-to-work solids but superelastic deformation, especially in a single-crystal-to-single-crystal manner, was not demonstrated for organic crystals so far. Here the authors demonstrate a single-crystal-to-single-crystal superplasticity in a crystal of N,N-dimethyl-4-nitroaniline.
- Satoshi Takamizawa
- , Yuichi Takasaki
- & Noriaki Ozaki
-
Article
| Open Access Metal-organic framework patterns and membranes with heterogeneous pores for flow-assisted switchable separations
Metal-organic framework patterns and membranes with heterogeneous pores for flow-assisted switchable separations
Tailoring MOFs to allow access of complex and large molecules is a challenging task due to their inherent microporous nature. Here the authors engineer meso- and macroporous MOF patterns and membranes via a mild decarboxylation applicable to different substrates, demonstrating their potential in macromolecule separations.
- Guan-Young Jeong
- , Ajay K. Singh
- & Dong-Pyo Kim
-
Article
| Open Access Strobilurin biosynthesis in Basidiomycete fungi
Strobilurin biosynthesis in Basidiomycete fungi
Strobilurins are fungal metabolites that inspired the creation of β-methoxyacrylate agricultural fungicides. Here, Nofiani et al. identify the strobilurin biosynthesis gene cluster, encoding a polyketide synthase as well as an FAD-dependent oxygenase for an oxidative rearrangement leading to β-methoxyacrylate formation.
- Risa Nofiani
- , Kate de Mattos-Shipley
- & Russell J. Cox
-
Article
| Open Access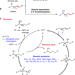 Asymmetric remote C-H borylation of internal alkenes via alkene isomerization
Asymmetric remote C-H borylation of internal alkenes via alkene isomerization
Sequential alkene isomerization/functionalization enables enantioselective transformations of remote C–H bonds. Here, the authors report a chiral cobalt catalytic system for the highly enantioselective, remote C–H borylation of internal alkenes via an isomerization/hydroboration sequence.
- Xu Chen
- , Zhaoyang Cheng
- & Zhan Lu
-
Article
| Open Access Stereoselective oxidative glycosylation of anomeric nucleophiles with alcohols and carboxylic acids
Stereoselective oxidative glycosylation of anomeric nucleophiles with alcohols and carboxylic acids
Glycosylation of partially protected sugars is usually limited by suboptimal regio- and stereo-selectivities. Here, the authors show a general oxidative glycosylation between anomeric stannanes with a nonprotected hydroxyl group and oxygen nucleophiles, additionally providing mechanistic insights into the origin of selectivity.
- Tianyi Yang
- , Feng Zhu
- & Maciej A. Walczak
-
Article
| Open Access H-bonded reusable template assisted para-selective ketonisation using soft electrophilic vinyl ethers
H-bonded reusable template assisted para-selective ketonisation using soft electrophilic vinyl ethers
Electrophilic acylation of arenes is largely limited to electron rich systems, non-polar medium and often displays moderate selectivity. Here, the authors show a directed para-selective ketonisation of arenes, overriding electronic bias and structural congestion, and apply it to the synthesis of bioactive compounds.
- Arun Maji
- , Amit Dahiya
- & Debabrata Maiti
-
Article
| Open Access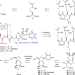 Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens
Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens
Albomycins are promising drug candidates for the treatment of bacterial infections. Here, the authors describe the total syntheses of albomycins δ1, δ2, and ε, and evaluate their antimicrobial activity, identifying albomycin δ2 as a strong agent against S. pneumoniae and S. aureus infections.
- Zihua Lin
- , Xiaobo Xu
- & Yun He
-
Article
| Open Access Synthesis of chiral α-trifluoromethyl alcohols and ethers via enantioselective Hiyama cross-couplings of bisfunctionalized electrophiles
Synthesis of chiral α-trifluoromethyl alcohols and ethers via enantioselective Hiyama cross-couplings of bisfunctionalized electrophiles
Compounds containing stereocenters bearing -CF3 groups, especially in proximity of heteroatoms, are of great interest for drug and agrochemicals development. Here, the authors report a nickel-catalyzed Hiyama cross-coupling methodology for the asymmetric synthesis of valuable alpha-trifluoromethyl alcohols and ethers.
- Andrii Varenikov
- & Mark Gandelman
-
Article
| Open Access Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles
Dehydrogenative reagent-free annulation of alkenes with diols for the synthesis of saturated O-heterocycles
Dehydrogenative annulation is a valuable approach to heterocycles, however, stoichiometric oxidants are often required. Here, the authors describe the electrochemical dehydrogenative annulation of diols and alkenes to generate dioxanes and dioxepanes under metal- and oxidant-free conditions.
- Chen-Yan Cai
- & Hai-Chao Xu
-
Article
| Open Access A general deoxygenation approach for synthesis of ketones from aromatic carboxylic acids and alkenes
A general deoxygenation approach for synthesis of ketones from aromatic carboxylic acids and alkenes
Synthesis of aryl ketones can be generally achieved via C–C coupling strategies. Here, the authors show a deoxygenative coupling of carboxylic acids and alkenes enabled by photoredox catalysis and report a wide scope of products, including bioactive and macrocyclic ketones.
- Muliang Zhang
- , Jin Xie
- & Chengjian Zhu
-
Article
| Open Access Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay
Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay
To date the carboacylation of alkenes has been reported only in single- and two-component methodologies. Here, the authors report a three-component nickel-catalyzed carboacylation of olefins which enables the rapid construction of ketones with high levels of complexity and diversity.
- Xian Zhao
- , Hai-Yong Tu
- & Lingling Chu
-
Article
| Open Access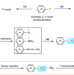 Formal group insertion into aryl C‒N bonds through an aromaticity destruction-reconstruction process
Formal group insertion into aryl C‒N bonds through an aromaticity destruction-reconstruction process
Activation of C–N bonds of anilines requires transformation of the amino group into a more reactive functionality. Here, the authors report an aromaticity destruction-reconstruction process that converts abundant anilines into valuable amines through group insertion into the C–N bond and benzylic C–H functionalization.
- Dandan Han
- , Qiuqin He
- & Renhua Fan
-
Article
| Open Access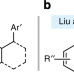 One-pot aminobenzylation of aldehydes with toluenes
One-pot aminobenzylation of aldehydes with toluenes
Amines are common targets in synthetic chemistry as they often display biological activity. Here, the authors report a one-pot aminobenzylation of aldehydes with readily available toluenes in presence of a sodium amide derivative and a cesium salt additive generating an array of valuable 1,2-diarylethylamine compounds.
- Zhiting Wang
- , Zhipeng Zheng
- & Patrick J. Walsh
-
Article
| Open Access Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)–H bonds
Metal-free alcohol-directed regioselective heteroarylation of remote unactivated C(sp3)–H bonds
Direct remote C–H functionalization of aliphatic alcohols via alkoxy radicals is largely unexplored. Here, the authors report the C(sp3)-heteroaryl bond formation in aliphatic alcohols mediated by alkoxy radicals formed with a hypervalent iodine reagent.
- Xinxin Wu
- , Hong Zhang
- & Chen Zhu
-
Article
| Open Access Enantioselective radical conjugate additions driven by a photoactive intramolecular iminium-ion-based EDA complex
Enantioselective radical conjugate additions driven by a photoactive intramolecular iminium-ion-based EDA complex
In enantioselective catalysis, electron donor–acceptor (EDA) complexes are usually photochemically activated in an intermolecular fashion. Here, the authors report the stereoselective conjugate addition to β-substituted cyclic enones proceeding without external photoredox catalysts via an intramolecular EDA complex.
- Zhong-Yan Cao
- , Tamal Ghosh
- & Paolo Melchiorre
-
Article
| Open Access An isolable catenane consisting of two Möbius conjugated nanohoops
An isolable catenane consisting of two Möbius conjugated nanohoops
Molecules exhibiting Möbius topology are fascinating but challenging synthetic targets. Here, the authors report the elegant synthesis and crystal structure of a catenane formed from two fully conjugated, interlocked Möbius nanohoops, and use theoretical calculations to understand its conformational stability and aromaticity.
- Yang-Yang Fan
- , Dandan Chen
- & Huan Cong
-
Article
| Open Access Iridium-catalyzed reductive Ugi-type reactions of tertiary amides
Iridium-catalyzed reductive Ugi-type reactions of tertiary amides
Chemical transformation of amides is normally occurring under harsh conditions. Here, the authors report a mild iridium-catalyzed reductive Ugi-type coupling of tertiary amides, isocyanides and (thio)acetic acid or trimethylsilyl azide to give homologous, bioactive amine products.
- Lan-Gui Xie
- & Darren J. Dixon
-
Article
| Open Access Remote C−H functionalization using radical translocating arylating groups
Remote C−H functionalization using radical translocating arylating groups
Selective remote functionalization of aliphatic C(sp3)−H bonds is highly challenging and often requires transition metals and/or directing groups. Here, the authors show the γ-arylation of aliphatic alcohols via a two-step radical translocation and subsequent radical aryl migration.
- Florian W. Friese
- , Christian Mück-Lichtenfeld
- & Armido Studer
-
Article
| Open Access Silver-catalyzed remote Csp3-H functionalization of aliphatic alcohols
Silver-catalyzed remote Csp3-H functionalization of aliphatic alcohols
Aliphatic alcohols usually require pre-activation for successful functionalization of their carbon chain. Here, the authors report a silver-catalyzed δ-selective functionalization of aliphatic alcohols via Csp3-H bond cleavage under mild conditions without the need of substrate pre-activation.
- Yuchao Zhu
- , Kaimeng Huang
- & Ning Jiao
-
Article
| Open Access Direct evidence for hula twist and single-bond rotation photoproducts
Direct evidence for hula twist and single-bond rotation photoproducts
Photoisomerization mechanisms govern important (bio)catalytic reactions and lie at the core of many functional materials. Here, the authors report a molecular setup that allows for the direct and separate observation of three fundamental photoreactions, namely the hula twist, single-bond rotation, as well as double-bond isomerization.
- Aaron Gerwien
- , Monika Schildhauer
- & Henry Dube

 Catalytic enantioselective oxidative coupling of saturated ethers with carboxylic acid derivatives
Catalytic enantioselective oxidative coupling of saturated ethers with carboxylic acid derivatives