Article
|
Open Access
Featured
-
-
Article
| Open Access Reconstitution of early paclitaxel biosynthetic network
Reconstitution of early paclitaxel biosynthetic network
Paclitaxel is an important anticancer drug whose biosynthetic pathway reconstruction is hindered by the propensity of heterologously expressed pathway cytochromes P450, including taxadiene 5α-hydroxylase (T5αH), to form multiple products. Here, the authors tune the promoter strength for T5αH expression in Nicotiana plants to increase the levels of paclitaxel precursor taxadien-5α-ol by three-fold and reconstitute the six step early biosynthetic pathway of paclitaxel.
- Jack Chun-Ting Liu
- , Ricardo De La Peña
- & Elizabeth S. Sattely
-
Article
| Open Access Elucidation of unusual biosynthesis and DnaN-targeting mode of action of potent anti-tuberculosis antibiotics Mycoplanecins
Elucidation of unusual biosynthesis and DnaN-targeting mode of action of potent anti-tuberculosis antibiotics Mycoplanecins
Mycoplanecins show promising activity against tuberculosis. Here, the authors identify and study mycoplanecins’ biosynthesis, antibacterial effects, and binding mechanism to DnaN, suggesting potential for fighting multidrug-resistant tuberculosis.
- Chengzhang Fu
- , Yunkun Liu
- & Rolf Müller
-
Article
| Open Access Modular assembly of an artificially concise biocatalytic cascade for the manufacture of phenethylisoquinoline alkaloids
Modular assembly of an artificially concise biocatalytic cascade for the manufacture of phenethylisoquinoline alkaloids
Plant alkaloids – an important class of pharmaceuticals - are still largely acquired through phytoextraction. Here, the authors develop an artificial and concise four-enzyme biocatalytic cascade for synthesizing various phenethylisoquinoline alkaloids from readily available starting materials.
- Yue Gao
- , Fei Li
- & Yijian Rao
-
Article
| Open Access A naturally occurring polyacetylene isolated from carrots promotes health and delays signatures of aging
A naturally occurring polyacetylene isolated from carrots promotes health and delays signatures of aging
Ameliorating or preventing signatures of aging in humans using natural compounds is an exciting area of research. Here the authors isolate a previously unknown phytochemical from carrots which activates defence mechanisms against oxidative stress and extends lifespan in worms, and improves glucose metabolism, promotes exercise capacity, and protects from frailty at higher age in mice.
- Carolin Thomas
- , Reto Erni
- & Michael Ristow
-
Article
| Open Access Discovery of type II polyketide synthase-like enzymes for the biosynthesis of cispentacin
Discovery of type II polyketide synthase-like enzymes for the biosynthesis of cispentacin
Type II polyketide synthases (PKSs) normally synthesize polycyclic aromatic compounds, but the potential for the synthesis of further diverse skeletons remains under investigated. Here, the authors report the discovery of the type II PKS machinery for the biosynthesis of a five-membered nonaromatic skeleton contained in the nonproteinogenic amino acid cispentacin and the plant toxin coronatine.
- Genki Hibi
- , Taro Shiraishi
- & Tomohisa Kuzuyama
-
Article
| Open Access Streptomyces alleviate abiotic stress in plant by producing pteridic acids
Streptomyces alleviate abiotic stress in plant by producing pteridic acids
Soil microbiota can increase crop resilience to abiotic stressors. Here the authors show that Streptomyces produce bioactive spiroketal polyketides to enhance plant growth under drought and salt stress.
- Zhijie Yang
- , Yijun Qiao
- & Ling Ding
-
Article
| Open Access Biosynthesis and engineering of the nonribosomal peptides with a C-terminal putrescine
Biosynthesis and engineering of the nonribosomal peptides with a C-terminal putrescine
Nonribosomal peptides have diverse bioactivities and can possess unusual moieties at their C-terminus, such as polyamines. In this study, the authors identify a class of dodecapeptides glidonins that feature diverse N-terminal modifications and a uniform putrescine moiety at the C-terminus, elucidate their biosynthesis, and introduce the putrescine into the C-terminus of other nonribosomal peptides.
- Hanna Chen
- , Lin Zhong
- & Xiaoying Bian
-
Article
| Open Access Triepoxide formation by a flavin-dependent monooxygenase in monensin biosynthesis
Triepoxide formation by a flavin-dependent monooxygenase in monensin biosynthesis
MonCI, a flavin-dependent monooxygenase, transforms all three C = C groups in the polyene substrate into epoxides during monensin A biosynthesis. Here, the authors present the structural basis for this enzyme’s regio- and stereoselective epoxidation activity.
- Qian Wang
- , Ning Liu
- & Chu-Young Kim
-
Article
| Open Access O-methyltransferase-like enzyme catalyzed diazo installation in polyketide biosynthesis
O-methyltransferase-like enzyme catalyzed diazo installation in polyketide biosynthesis
Diazo compounds, such as kinamycin, are rare bioactive natural products whose assembly has been extensively studied, but the formation of the diazo group is elusive. Here, the authors report O-methyltransferase-like protein, AlpH, which is responsible for the l-glutamylhydrazine incorporation in kinamycin biosynthesis.
- Yuchun Zhao
- , Xiangyang Liu
- & Ming Jiang
-
Article
| Open Access An unconventional proanthocyanidin pathway in maize
An unconventional proanthocyanidin pathway in maize
Maize has an unconventional proanthocyanidin (PA) biosynthetic pathway which produces rare stereoisomers of PA monomers and dimers, highlighting an opportunity for further improving the nutritional value of maize.
- Nan Lu
- , Ji Hyung Jun
- & Richard A. Dixon
-
Article
| Open Access HypoRiPPAtlas as an Atlas of hypothetical natural products for mass spectrometry database search
HypoRiPPAtlas as an Atlas of hypothetical natural products for mass spectrometry database search
A gap exists between large-scale genome mining and mass spectral datasets for natural product discovery. Here the authors bridge the gap by developing HypoRiPPAtlas, an Atlas of hypothetical natural product structures, which is ready-to-use for in silico database search of tandem mass spectra.
- Yi-Yuan Lee
- , Mustafa Guler
- & Hosein Mohimani
-
Article
| Open Access Molecular insights into the catalytic promiscuity of a bacterial diterpene synthase
Molecular insights into the catalytic promiscuity of a bacterial diterpene synthase
Diterpene synthase VenA catalyses the synthesis of venezuelaene A with a unique 5-5-6-7 tetracyclic skeleton from geranylgeranyl pyrophosphate. Here, the authors report crystal structures of apo- and holo-VenA, provide mechanistic insights into its substrate selectivity and promiscuity, and engineer VenA into a sesterterpene synthase.
- Zhong Li
- , Lilan Zhang
- & Shengying Li
-
Article
| Open Access Discovery of extended product structural space of the fungal dioxygenase AsqJ
Discovery of extended product structural space of the fungal dioxygenase AsqJ
The fungal dioxygenase AsqJ catalyses the conversion of benzo[1,4]diazepine-2,5-diones into quinolone antibiotics, and can also catalyse the production of quinazolinones. Here, the authors perform comprehensive substrate promiscuity mapping of AsqJ revealing its large tolerance towards various substrates, giving biocatalytic access to a plethora of biomedically valuable heterocyclic frameworks.
- Manuel Einsiedler
- & Tobias A. M. Gulder
-
Article
| Open Access A flavin-monooxygenase catalyzing oxepinone formation and the complete biosynthesis of vibralactone
A flavin-monooxygenase catalyzing oxepinone formation and the complete biosynthesis of vibralactone
Vibralactone is a strong lipase inhibitor and a bicyclic β-lactone containing an oxepinone ring, whose biosynthetic construction was unknown. Here the authors identify an oxepinone-building flavin monooxygenase VibO that is involved in the biosynthesis of vibralactone, and determine its X-ray crystal structure.
- Ke-Na Feng
- , Yue Zhang
- & Ying Zeng
-
Article
| Open Access Discovery and biosynthesis of tricyclic copper-binding ribosomal peptides containing histidine-to-butyrine crosslinks
Discovery and biosynthesis of tricyclic copper-binding ribosomal peptides containing histidine-to-butyrine crosslinks
Cyclic peptides are important bioactive compounds and drugs, synthesised by enzymatic side-chain macrocyclization of ribosomal peptides, which rarely involves histidine residues. Here, the authors report the discovery and biosynthesis of tricyclic lanthipeptide noursin, constrained by a tri amino acid labionin crosslink and histidine-to-butyrine crosslink, which is important for copper binding of noursin.
- Yuqing Li
- , Yeying Ma
- & Huan Wang
-
Article
| Open Access Unveiling an indole alkaloid diketopiperazine biosynthetic pathway that features a unique stereoisomerase and multifunctional methyltransferase
Unveiling an indole alkaloid diketopiperazine biosynthetic pathway that features a unique stereoisomerase and multifunctional methyltransferase
Diketopiperazine (DKP) natural products have diverse structures and biological functions. Here, the authors elucidate the biosynthetic pathway for indole alkaloid DKP nocardioazine B which includes DKP stereoisomerization by an unusual aspartate/glutamate racemase homolog and N- and C-methylation by a dual function methyltransferase.
- Garrett Deletti
- , Sajan D. Green
- & Amy L. Lane
-
Article
| Open Access Collateral sensitivity profiling in drug-resistant Escherichia coli identifies natural products suppressing cephalosporin resistance
Collateral sensitivity profiling in drug-resistant Escherichia coli identifies natural products suppressing cephalosporin resistance
Collateral sensitivity (CS), whereby resistance to one drug is accompanied by increased sensitivity to another, provides new opportunities for antimicrobial drug discovery. Here, Liu et al. screen large chemical libraries across 29 drug-resistant E. coli strains to identify multiple CS interactions, including natural products with potent CS activities against cephalosporin-resistant strains.
- Dennis Y. Liu
- , Laura Phillips
- & Roger G. Linington
-
Perspective
| Open Access A roadmap to establish a comprehensive platform for sustainable manufacturing of natural products in yeast
A roadmap to establish a comprehensive platform for sustainable manufacturing of natural products in yeast
Yeasts, Saccharomyces cerevisiae and Pichia pastoris, are promising chassis for the production of nature products (NPs). Here, the author discusses establishing a comprehensive platform for sustainable production of NPs via system-associated optimization at genetics, temporal controllers, productivity screening, and scalability levels.
- Gita Naseri
-
Article
| Open Access Genome mining unveils a class of ribosomal peptides with two amino termini
Genome mining unveils a class of ribosomal peptides with two amino termini
RiPP discovery has expanded the scope of post-translational modification chemistry, but genome mining of RiPP classes remains an unsolved challenge. Here, the authors employed bioinformatics and synthetic biology approaches to discover and characterize an unknown class of RiPPs, defined by an unusual amino-modified C-terminus.
- Hengqian Ren
- , Shravan R. Dommaraju
- & Huimin Zhao
-
Article
| Open Access Decrypting the programming of β-methylation in virginiamycin M biosynthesis
Decrypting the programming of β-methylation in virginiamycin M biosynthesis
Biosynthesis of complex polyketides by polyketide synthases often relies on trans-acting enzymes to modify the intermediates. Here, the authors elucidate how β-methylation enzymes identify their substrates. The recognition is imperfect, resulting in a doubly β-methylated virginiamycin derivative.
- Sabrina Collin
- , Russell J. Cox
- & Arnaud Gruez
-
Article
| Open Access Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition
Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition
Intermodular recognition in polyketide synthase (PKS) is a key prerequisite for catalysis and assembly-line engineering. Here, the authors present a specific domain interaction between modules and an evolution-oriented strategy for PKS engineering derived from the enoylreduction module of azalomycin F.
- Guifa Zhai
- , Yan Zhu
- & Yuhui Sun
-
Article
| Open Access Uncovering a miltiradiene biosynthetic gene cluster in the Lamiaceae reveals a dynamic evolutionary trajectory
Uncovering a miltiradiene biosynthetic gene cluster in the Lamiaceae reveals a dynamic evolutionary trajectory
A diterpenoid biosynthetic gene cluster (BGC) has been identified in a few species in the Lamiaceae (mint) family, but its origin and evolution remain unclear. Here, the authors report assembly of genomes of three species within the family and reveal the dynamic evolutionary trajectory of the BGC.
- Abigail E. Bryson
- , Emily R. Lanier
- & Björn Hamberger
-
Article
| Open Access Annotation of natural product compound families using molecular networking topology and structural similarity fingerprinting
Annotation of natural product compound families using molecular networking topology and structural similarity fingerprinting
Comparing experimental mass spectra to reference spectra can enable natural product identification, but these spectral libraries are often incomplete and not universally applicable. Here, the authors present SNAP-MS, a tool that allows assigning compound families without experimental or calculated reference spectra.
- Nicholas J. Morehouse
- , Trevor N. Clark
- & Roger G. Linington
-
Article
| Open Access Biosynthesis of mushroom-derived type II ganoderic acids by engineered yeast
Biosynthesis of mushroom-derived type II ganoderic acids by engineered yeast
The biosynthetic pathway of type II ganoderic acids (GAs) in Ganoderma lucidum, a traditional medicinal mushroom, is unknown. Here, the authors assemble the genome of type II GAs accumulating accession, identify CYPs involving in type II GAs biosynthesis, and achieve their production in engineered baker’s yeast.
- Wei Yuan
- , Chenjian Jiang
- & Han Xiao
-
Article
| Open Access Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis
Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis
4(3H)-quinazolinone is the core scaffold in more than 200 natural alkaloids and numerous drugs. Here, the authors show an alternative assembly machinery for 4(3H)-quinazolinone mainly includes a two-module NRPS catalysing tripeptide formation, unusual N-3 original sources and an α-KGD catalysing the C-N bond oxidative cleavage of a tripeptide to form a dipeptide.
- Xi-Wei Chen
- , Li Rao
- & Yi Zou
-
Article
| Open Access The occurrence of ansamers in the synthesis of cyclic peptides
The occurrence of ansamers in the synthesis of cyclic peptides
The occurrence of isomers of the bicyclic octapeptide α-amanitin, which presents a macrolactam with a tryptathionine cross-link forming a handle, has been reported under the term of atropoisomers. Here, the authors synthesize its analogs and analyse their isomers, proposing and describing for them the term ansamer.
- Guiyang Yao
- , Simone Kosol
- & Roderich D. Süssmuth
-
Article
| Open Access Oxygen mediated oxidative couplings of flavones in alkaline water
Oxygen mediated oxidative couplings of flavones in alkaline water
Catalysed oxidative C-C bond formation reactions are important in the synthesis of natural products, but poorly tolerated by polyphenolic flavones. Here the authors report the reactivity of molecular oxygen in alkaline water without added catalyst for the synthesis of a collection of flavone dimers and trimers.
- Xin Yang
- , Sophie Hui Min Lim
- & Dejian Huang
-
Article
| Open Access A scalable platform to discover antimicrobials of ribosomal origin
A scalable platform to discover antimicrobials of ribosomal origin
Ribosomally synthesized and post-translationally modified peptides are a source of antimicrobials. Here, the authors report a platform for the rapid evaluation and characterization of biosynthetic gene clusters that enables the identification of 30 structurally diverse modified peptides, including three showing antimicrobial activities.
- Richard S. Ayikpoe
- , Chengyou Shi
- & Huimin Zhao
-
Article
| Open Access Chimeric natural products derived from medermycin and the nature-inspired construction of their polycyclic skeletons
Chimeric natural products derived from medermycin and the nature-inspired construction of their polycyclic skeletons
Nonenzymatic reactions play an important part in the formation of some natural products possessing complex skeletons. Here, the authors report the discovery of eight chimeric medermycin-type natural products and their nonenzymatic construction.
- Shupeng Yin
- , Zhi Liu
- & Peng Fu
-
Article
| Open Access A ribosomally synthesised and post-translationally modified peptide containing a β-enamino acid and a macrocyclic motif
A ribosomally synthesised and post-translationally modified peptide containing a β-enamino acid and a macrocyclic motif
The chemical diversity of peptides from ribosomal origin is a growing field of research. Here, the authors report the discovery, genomic and biosynthetic investigations of kintamdin, a ribosomally synthesized and post-translationally modified peptides featuring a beta-enamino acid and a bis-thioether macrocyclic motif.
- Shan Wang
- , Sixing Lin
- & Hai Deng
-
Article
| Open Access Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide
Tripterygium wilfordii cytochrome P450s catalyze the methyl shift and epoxidations in the biosynthesis of triptonide
How triptonide is made in the medicinal plant Tripterygium wilfordii is largely unknown. Here, the authors report the identification and characterization of a suite of cytochrome P450s and show their function in catalyzing the formation of triptonide from miltriadiene in tobacco and baker’s yeast.
- Nikolaj Lervad Hansen
- , Louise Kjaerulff
- & Johan Andersen-Ranberg
-
Article
| Open Access Biocatalytic routes to stereo-divergent iridoids
Biocatalytic routes to stereo-divergent iridoids
Iridoid compounds are an important class of natural products. Here, the authors report on the discovery and engineering of nepetalactol-related short chain reductases and their application for the biosynthesis of nepetalactol or nepetalactone stereoisomers, as a versatile system for the production of the iridoid natural product scaffold.
- Néstor J. Hernández Lozada
- , Benke Hong
- & Sarah E. O’Connor
-
Article
| Open Access Native metabolomics identifies the rivulariapeptolide family of protease inhibitors
Native metabolomics identifies the rivulariapeptolide family of protease inhibitors
Bioactivity-guided isolation of specialized metabolites is an iterative process. Here, the authors demonstrate a native metabolomics approach that allows for fast screening of complex metabolite extracts against a protein of interest and simultaneous structure annotation.
- Raphael Reher
- , Allegra T. Aron
- & Daniel Petras
-
Article
| Open Access A cryptic third active site in cyanophycin synthetase creates primers for polymerization
A cryptic third active site in cyanophycin synthetase creates primers for polymerization
Cyanophycin synthetase CphA1 polymerizes Asp and Arg into the nitrogen reserve polymer cyanophycin using two active sites. Sharon et al. show CphA1 has a cryptic 3rd active site that cleaves cyanophycin into primers for self-sufficient biosynthesis.
- Itai Sharon
- , Sharon Pinus
- & T. Martin Schmeing
-
Article
| Open Access A GPCR-based yeast biosensor for biomedical, biotechnological, and point-of-use cannabinoid determination
A GPCR-based yeast biosensor for biomedical, biotechnological, and point-of-use cannabinoid determination
GPCRs are used for diverse sensing in eukaryotes. Here the authors use GPCRs to construct yeast-based biosensors, focussing on cannabinoids, and use these to screen agonists and antagonists, as well as generate a portable detection device.
- Karel Miettinen
- , Nattawat Leelahakorn
- & Sotirios C. Kampranis
-
Article
| Open Access Complete biosynthetic pathway to the antidiabetic drug acarbose
Complete biosynthetic pathway to the antidiabetic drug acarbose
The market demand for acarbose, a drug used for treatment of patients affected by type-2 diabetes, has increased. In this article, the authors report the acarbose complete biosynthetic pathway, clarifying previously unknown steps and identifying a pseudoglycosyltransferase enzyme, AcbS, a homologue of AcbI that catalyzes the formation of a non-glycosidic C-N bond.
- Takeshi Tsunoda
- , Arash Samadi
- & Taifo Mahmud
-
Article
| Open Access Molecular basis of antibiotic self-resistance in a bee larvae pathogen
Molecular basis of antibiotic self-resistance in a bee larvae pathogen
The authors show that the N-acetyltransferase PamZ acts as a self-resistance factor disabling the antibacterial paenilamicin that is produced by the honey bee larvae pathogen Paenibacillus larvae.
- Tam Dang
- , Bernhard Loll
- & Roderich D. Süssmuth
-
Article
| Open Access Correlational networking guides the discovery of unclustered lanthipeptide protease-encoding genes
Correlational networking guides the discovery of unclustered lanthipeptide protease-encoding genes
Many natural product biosynthetic gene clusters are found not to harbor a full set of necessary genes and must utilize enzymes encoded elsewhere (hidden) in the genome for biosynthesis. Here, the authors establish a global correlation network bridging the gap between lanthipeptide precursors and hidden proteases.
- Dan Xue
- , Ethan A. Older
- & Jie Li
-
Article
| Open Access Reconstituting the complete biosynthesis of D-lysergic acid in yeast
Reconstituting the complete biosynthesis of D-lysergic acid in yeast
The ergot alkaloids are a class of natural products known for their pharmacologically privileged molecular structure that are used in the treatment of neurological ailments. Here the authors report on the production of the ergot (fungus)-derived therapeutic precursor, D-lysergic acid (DLA), in baker’s yeast.
- Garrett Wong
- , Li Rong Lim
- & Wen Shan Yew
-
Article
| Open Access Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica
Removal of lycopene substrate inhibition enables high carotenoid productivity in Yarrowia lipolytica
Substrate inhibition has not been widely studied in the context of synthetic biology and metabolic engineering. Here, the authors report removal of lycopene substrate inhibition by two different strategies and enable high carotenoid productivity in Yarrowia lipolytica.
- Yongshuo Ma
- , Nian Liu
- & Gregory Stephanopoulos
-
Article
| Open Access Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module
Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module
Nonribosomal peptide synthetases work with additional enzymes to synthesise secondary metabolites and therapeutics. Here, the authors explore bacillamide D synthesis and show the oxidase action is done while the intermediate is attached to the synthetase and replicate this with an oxidase bound synthetase for bioengineering applications.
- Camille Marie Fortinez
- , Kristjan Bloudoff
- & T. Martin Schmeing
-
Article
| Open Access Berberine bridge enzyme-like oxidase-catalysed double bond isomerization acts as the pathway switch in cytochalasin synthesis
Berberine bridge enzyme-like oxidase-catalysed double bond isomerization acts as the pathway switch in cytochalasin synthesis
Cytochalasans are a large family of fungal polyketide-nonribosomal peptide hybrid natural products that exhibit important pharmaceutical activities, but the mechanism of conversion of the monocytochalasans to the polycyclic, fused analogues is unclear. Here the authors reconstitute the core backbone of the cytochalasin family and describe an oxidase that catalyzes an unusual double-bond isomerization reaction.
- Jin-Mei Zhang
- , Xuan Liu
- & Yi Zou
-
Article
| Open Access Structural evolution of a DNA repair self-resistance mechanism targeting genotoxic secondary metabolites
Structural evolution of a DNA repair self-resistance mechanism targeting genotoxic secondary metabolites
Microbial DNA glycosylases associated with the biosynthesis of DNA-damaging antibiotics have evolved self-resistance for their cognate natural products. Here, the authors provide evidence that cellular self-resistance is enabled by reduced affinity of the glycosylases for the excision products of the corresponding DNA lesions.
- Elwood A. Mullins
- , Jonathan Dorival
- & Brandt F. Eichman
-
Article
| Open Access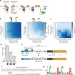 Selection for constrained peptides that bind to a single target protein
Selection for constrained peptides that bind to a single target protein
Peptide secondary metabolites have a diverse range of functions. Here the authors present a method to design and screen a large library of modified peptides in E. coli against a target of interest.
- Andrew M. King
- , Daniel A. Anderson
- & Christopher A. Voigt
-
Article
| Open Access Thiocysteine lyases as polyketide synthase domains installing hydropersulfide into natural products and a hydropersulfide methyltransferase
Thiocysteine lyases as polyketide synthase domains installing hydropersulfide into natural products and a hydropersulfide methyltransferase
Enzymes installing an intact hydropersulfide (-SSH) group into natural products have so far not been identified. Here, the authors report the characterization of an S-adenosyl methionine-dependent hydropersulfide methyltransferase (GnmP) for guangnanmycin biosynthesis, and identification of three SH domains within several NRPS-PKS assembly lines as thiocysteine lyases.
- Song Meng
- , Andrew D. Steele
- & Ben Shen
-
Article
| Open Access Conformational rearrangements enable iterative backbone N-methylation in RiPP biosynthesis
Conformational rearrangements enable iterative backbone N-methylation in RiPP biosynthesis
Borosins are ribosomally encoded and posttranslationally modified peptide (RiPP) natural products featuring amide-backbone α-N-methylation. Here, the authors report the discovery and characterization of type IV borosin ‘split’ pathways encoding distinct, separate α-N-methyltransferases and precursor peptide substrates.
- Fredarla S. Miller
- , Kathryn K. Crone
- & Michael F. Freeman
-
Article
| Open Access Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan
Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan
The only known animal polyketide-nonribosomal peptides, the nemamides, are biosynthesized by two megasynthetases in the canal-associated neurons (CANs) of C. elegans. Here, the authors map the biosynthetic roles of individual megasynthetase domains and identify additional enzymes in the CANs required for nemamide biosynthesis.
- Likui Feng
- , Matthew T. Gordon
- & Rebecca A. Butcher
-
Article
| Open Access Rational construction of genome-reduced Burkholderiales chassis facilitates efficient heterologous production of natural products from proteobacteria
Rational construction of genome-reduced Burkholderiales chassis facilitates efficient heterologous production of natural products from proteobacteria
An efficient chassis for heterologous expression of biosynthetic gene clusters (BGCs) from Gram-negative bacteria is still unavailable. Here, the authors report rational construction of genome-reduced Burkholderials chassis to facilitate production of a class of new compounds by expressing BGC from Chitinimonas koreensis.
- Jiaqi Liu
- , Haibo Zhou
- & Xiaoying Bian
-
Article
| Open Access Initiating polyketide biosynthesis by on-line methyl esterification
Initiating polyketide biosynthesis by on-line methyl esterification
Aurantinins are polyketides with unusual connectivities and broad antibacterial activity. Here the authors show the biosynthesis of aurantinins, which proceeds via an on-line methyl esterification at the terminus that enables the iterative chain elongations prior to condensation and cyclization.
- Pengwei Li
- , Meng Chen
- & Yihua Chen

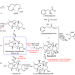 Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol
Biosynthesis of the highly oxygenated tetracyclic core skeleton of Taxol