Featured
-
-
Article
| Open Access High resolution cryo-EM and crystallographic snapshots of the actinobacterial two-in-one 2-oxoglutarate dehydrogenase
High resolution cryo-EM and crystallographic snapshots of the actinobacterial two-in-one 2-oxoglutarate dehydrogenase
The 2-oxoglutarate dehydrogenase complex (ODH) is a textbook example of multienzymatic machinery. Here, the authors report the structural and regulatory properties of the Actinobacterial enzyme OdhA, a fusion of two ODH components acting in a supercomplex with pyruvate dehydrogenase.
- Lu Yang
- , Tristan Wagner
- & Marco Bellinzoni
-
Article
| Open Access The Ycf48 accessory factor occupies the site of the oxygen-evolving manganese cluster during photosystem II biogenesis
The Ycf48 accessory factor occupies the site of the oxygen-evolving manganese cluster during photosystem II biogenesis
The Ycf48 accessory factor is important for the assembly and repair of the photosystem II (PSII) complex of oxygenic photosynthesis. Here, the authors show that Ycf48 occupies the binding site of the oxygen evolving Mn cluster early in PSII biogenesis.
- Ziyu Zhao
- , Irene Vercellino
- & Josef Komenda
-
Article
| Open Access Architecture of the human G-protein-methylmalonyl-CoA mutase nanoassembly for B12 delivery and repair
Architecture of the human G-protein-methylmalonyl-CoA mutase nanoassembly for B12 delivery and repair
B12-dependent human methylmalonly-CoA mutase (MMUT) requires the chaperone MMAA. The authors report the crystal structure of MMUT-MMAA, which shows a MMAA-driven conformational change in MMUT involved n B12 loading and repair and helps explain the effects of disease-causing MMAA-MMUT interface mutations.
- Romila Mascarenhas
- , Markus Ruetz
- & Ruma Banerjee
-
Article
| Open Access Atomic model for core modifying region of human fatty acid synthase in complex with Denifanstat
Atomic model for core modifying region of human fatty acid synthase in complex with Denifanstat
Here, the authors develop a protein engineering method that enables high-resolution structural biology study of human fatty acid synthase. Using this technique, they uncover unique structural features of the enzyme and the mechanism of its inhibition by an anticancer drug Denifanstat.
- S. M. Naimul Hasan
- , Jennifer W. Lou
- & Mohammad T. Mazhab-Jafari
-
Article
| Open Access Decrypting the programming of β-methylation in virginiamycin M biosynthesis
Decrypting the programming of β-methylation in virginiamycin M biosynthesis
Biosynthesis of complex polyketides by polyketide synthases often relies on trans-acting enzymes to modify the intermediates. Here, the authors elucidate how β-methylation enzymes identify their substrates. The recognition is imperfect, resulting in a doubly β-methylated virginiamycin derivative.
- Sabrina Collin
- , Russell J. Cox
- & Arnaud Gruez
-
Article
| Open Access Structural remodelling of the carbon–phosphorus lyase machinery by a dual ABC ATPase
Structural remodelling of the carbon–phosphorus lyase machinery by a dual ABC ATPase
Here, authors analyse the structural organisation of the large carbon-phosphorus lyase enzyme from bacteria using electron microscopy and discover that it contains two ATP-binding cassette dimers of PhnK and PhnL and opens upon ATP hydrolysis.
- Søren K. Amstrup
- , Sui Ching Ong
- & Ditlev E. Brodersen
-
Article
| Open Access Genomics and biochemical analyses reveal a metabolon key to β-L-ODAP biosynthesis in Lathyrus sativus
Genomics and biochemical analyses reveal a metabolon key to β-L-ODAP biosynthesis in Lathyrus sativus
Grass pea is a multi-stress tolerant orphan crop and developing cultivars with decreased accumulation of the neurotoxin β-L-oxalyl-2,3-diaminopropionic acid (β-L-ODAP) is one of its breeding objectives. Here, the authors assemble its genome and reveal genes involved in the biosynthesis of β-L-ODAP.
- Anne Edwards
- , Isaac Njaci
- & Peter M. F. Emmrich
-
Article
| Open Access Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition
Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition
Intermodular recognition in polyketide synthase (PKS) is a key prerequisite for catalysis and assembly-line engineering. Here, the authors present a specific domain interaction between modules and an evolution-oriented strategy for PKS engineering derived from the enoylreduction module of azalomycin F.
- Guifa Zhai
- , Yan Zhu
- & Yuhui Sun
-
Article
| Open Access A gene cluster in Ginkgo biloba encodes unique multifunctional cytochrome P450s that initiate ginkgolide biosynthesis
A gene cluster in Ginkgo biloba encodes unique multifunctional cytochrome P450s that initiate ginkgolide biosynthesis
Although ginkgo terpenoids have been studied extensively for their pharmaceutical properties, knowledge on their biosynthesis remains limited. Here, the authors identify five multifunctional cytochrome P450s that catalyze the generation of the tert-butyl group and one of the lactone rings towards the biosynthesis of ginkgolides.
- Victor Forman
- , Dan Luo
- & Irini Pateraki
-
Article
| Open Access Regulated degradation of HMG CoA reductase requires conformational changes in sterol-sensing domain
Regulated degradation of HMG CoA reductase requires conformational changes in sterol-sensing domain
HMG-CoA reductase (HMGCR) is regulated by UBIAD1 and Insigs and initializes cholesterol synthesis. Here authors show that the sterol sensing domain of HMGCR undergoes conformational changes to regulate its degradation via binding its protein modulators.
- Hongwen Chen
- , Xiaofeng Qi
- & Xiaochun Li
-
Article
| Open Access Engineering substrate specificity of HAD phosphatases and multienzyme systems development for the thermodynamic-driven manufacturing sugars
Engineering substrate specificity of HAD phosphatases and multienzyme systems development for the thermodynamic-driven manufacturing sugars
Haloacid dehalogenase-like phosphatases are widespread across all domains of life and play a crucial role in the regulation of levels of sugar phosphate metabolites in cells. The authors report on the structure-guided engineering of phosphatases for dedicated substrate specificity for the conversion of sucrose and starch into fructose and mannose.
- Chaoyu Tian
- , Jiangang Yang
- & Yanhe Ma
-
Article
| Open Access A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase
A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase
The drug AT-527 targets the SARS-CoV-2 replication machinery. Here the authors use Cryo-EM to show how AT-527 inhibits SARS-CoV-2 polymerase by acting as an immediate RNA chain terminator and stably binding in a NiRAN active-site pocket; impeding an essential nucleotide-transfer activity.
- Ashleigh Shannon
- , Véronique Fattorini
- & Bruno Canard
-
Article
| Open Access Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module
Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module
Nonribosomal peptide synthetases work with additional enzymes to synthesise secondary metabolites and therapeutics. Here, the authors explore bacillamide D synthesis and show the oxidase action is done while the intermediate is attached to the synthetase and replicate this with an oxidase bound synthetase for bioengineering applications.
- Camille Marie Fortinez
- , Kristjan Bloudoff
- & T. Martin Schmeing
-
Article
| Open Access Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins
Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins
Genetic engineering of the type I polyketide synthases (PKSs) to produce desirable analogous remains largely inefficient. Here, the authors leverage multiple approaches to delete seven internal modules from the stambomycin PKS and generate 37-membered mini-stambomycin macrolactones.
- Li Su
- , Laurence Hôtel
- & Kira J. Weissman
-
Article
| Open Access Cryo-EM snapshots of a native lysate provide structural insights into a metabolon-embedded transacetylase reaction
Cryo-EM snapshots of a native lysate provide structural insights into a metabolon-embedded transacetylase reaction
How is acetyl-CoA produced in the context of the endogenous, eukaryotic pyruvate dehydrogenase complex metabolon? Here the authors dissect the embedded transacetylase reaction through biochemical, cryo-EM, HADDOCKing and molecular dynamics methods.
- Christian Tüting
- , Fotis L. Kyrilis
- & Panagiotis L. Kastritis
-
Article
| Open Access AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway
AGPAT2 interaction with CDP-diacylglycerol synthases promotes the flux of fatty acids through the CDP-diacylglycerol pathway
AGPATs (1-acylglycerol-3-phosphate O-acyltransferases) catalyze the acylation of lysophosphatidic acid to form phosphatidic acid (PA), a key step in the synthesis of all glycerolipids. Here, the authors show that AGPAT2 and CDP-DAG synthases (CDS1 and CDS2) form functional complexes that promote further conversion of PA along the CDP-DAG pathway of phospholipid synthesis.
- Hoi Yin Mak
- , Qian Ouyang
- & Hongyuan Yang
-
Article
| Open Access Gene editing enables rapid engineering of complex antibiotic assembly lines
Gene editing enables rapid engineering of complex antibiotic assembly lines
Engineering biosynthetic assembly lines is a powerful path to new natural products but is challenging with current methods. Here the authors use CRISPR-Cas9 to exchange subdomains within NRPS to alter substrate selectivity.
- Wei Li Thong
- , Yingxin Zhang
- & Jason Micklefield
-
Article
| Open Access Hakai is required for stabilization of core components of the m6A mRNA methylation machinery
Hakai is required for stabilization of core components of the m6A mRNA methylation machinery
The E3 ligase Hakai can interact with the m6A methylation machinery but its function is still unclear. Here, the authors show that Hakai is a conserved component of the m6A methyltransferase complex and provide functional and molecular insights into its role in regulating m6A levels in Drosophila.
- Praveen Bawankar
- , Tina Lence
- & Jean-Yves Roignant
-
Article
| Open Access Structural insight on assembly-line catalysis in terpene biosynthesis
Structural insight on assembly-line catalysis in terpene biosynthesis
Substrate channeling can improve biosynthetic efficiency and has been implicated in the reactions of fusicoccadiene synthase. Here, the authors analyze this bifunctional enzyme complex by cryoEM, cross-linking MS and integrative modeling, providing structural insights into how substrate channeling is achieved.
- Jacque L. Faylo
- , Trevor van Eeuwen
- & David W. Christianson
-
Article
| Open Access A metal ion orients SARS-CoV-2 mRNA to ensure accurate 2′-O methylation of its first nucleotide
A metal ion orients SARS-CoV-2 mRNA to ensure accurate 2′-O methylation of its first nucleotide
The SARS-CoV-2 nsp16/nsp10 enzyme complex methylates the 2′-OH of the first nucleotide of the viral mRNA, converting the Cap-0 to Cap-1, which helps the virus to evade immune surveillance in the host cell. Here, the authors present the crystal structure of SARS-CoV-2 nsp16/nsp10 with the bound Cap-1 RNA nucleotide product and a post-release SAH containing structure.
- Thiruselvam Viswanathan
- , Anurag Misra
- & Yogesh K. Gupta
-
Article
| Open Access Structural coordination between active sites of a CRISPR reverse transcriptase-integrase complex
Structural coordination between active sites of a CRISPR reverse transcriptase-integrase complex
In some systems, a single protein comprising reverse transcriptase (RT), integrase and maturase enables concerted sequence integration and crRNA production. Here, analyses including the structure of a Cas6-RT-Cas1—Cas2 complex suggest coordination between all three active sites and capacity to acquire CRISPR sequences from RNA and DNA substrates.
- Joy Y. Wang
- , Christopher M. Hoel
- & Jennifer A. Doudna
-
Article
| Open Access Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity
Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity
Non-ribosomal peptide synthetases (NRPSs) are multi-modular enzymes assembling complex natural products. Here, the structures of a Thermobifida fusca NRPS condensation domain bound to the substrate-bearing peptidyl carrier protein (PCP) domain provide insight into the mechanisms of substrate selectivity and engagement within the catalytic pocket.
- Thierry Izoré
- , Y. T. Candace Ho
- & Max J. Cryle
-
Article
| Open Access In vivo and in vitro reconstitution of unique key steps in cystobactamid antibiotic biosynthesis
In vivo and in vitro reconstitution of unique key steps in cystobactamid antibiotic biosynthesis
The biosynthetic pathway leading to the production of cystobactamids, a group of myxobacteria-derived topoisomerase inhibitors with potent anti-Gram-negative activity, remains unclear. Here, the authors report in vivo and in vitro evidence for unique steps in cystobactamid biosynthesis.
- Sebastian Groß
- , Bastien Schnell
- & Rolf Müller
-
Article
| Open Access Cryo-EM structures of engineered active bc1-cbb3 type CIII2CIV super-complexes and electronic communication between the complexes
Cryo-EM structures of engineered active bc1-cbb3 type CIII2CIV super-complexes and electronic communication between the complexes
Respiratory chains generate the proton motive force used for ATP synthesis. Cryo-EM structures of functional respiratory CIII2CIV supercomplex and native CIII2 from Rhodobacter capsulatus provide insight into CIII2CIV assembly and respiratory electron transport pathways in Gram-negative bacteria.
- Stefan Steimle
- , Trevor van Eeuwen
- & Fevzi Daldal
-
Article
| Open Access Structural basis for the biosynthesis of lovastatin
Structural basis for the biosynthesis of lovastatin
Biosynthesis of the statin precursor lovastatin depends on the LovB–LovC megasynthase complex. Here, the authors present cryoEM structures of LovB–LovC and core LovB, providing structural insights into the catalytic cycle underlying lovastatin production.
- Jialiang Wang
- , Jingdan Liang
- & Zhijun Wang
-
Article
| Open Access Metabolic regulation of telomere silencing by SESAME complex-catalyzed H3T11 phosphorylation
Metabolic regulation of telomere silencing by SESAME complex-catalyzed H3T11 phosphorylation
Pyruvate kinase phosphorylates histone H3T11 (H3pT11) and represses gene expression by forming a large complex SESAME (Serine-responsive SAM-containing Metabolic Enzyme). Here the authors show that SESAME-catalyzed H3pT11 regulates telomere silencing by promoting Sir2 binding at telomeres and preventing autophagy-mediated Sir2 degradation.
- Shihao Zhang
- , Xilan Yu
- & Shanshan Li
-
Article
| Open Access Structure of a full-length bacterial polysaccharide co-polymerase
Structure of a full-length bacterial polysaccharide co-polymerase
Lipopolysaccharides, important components of the bacterial cell envelope, are synthesized at the inner membrane by the Wzx/Wzy-dependent assembly pathway. A cryo-EM structure of an intact E. coli WzzB, the polysaccharide co-polymerase component of this pathway, reveals details of the transmembrane, cytoplasmic domains and a conserved a proline-rich segment proximal to the C-terminal transmembrane helix.
- Benjamin Wiseman
- , Ram Gopal Nitharwal
- & Martin Högbom
-
Article
| Open Access Efficient rational modification of non-ribosomal peptides by adenylation domain substitution
Efficient rational modification of non-ribosomal peptides by adenylation domain substitution
Non-ribosomal peptide synthases are multimodular enzymes comprised of adenylation (A), condensation (C) and thiolation domains. Here, the authors show that non-ribosomal peptides can be generated solely by A domain substitutions, providing evidence that the postulated substrate specifying role of C-domains may be rare in nature.
- Mark J. Calcott
- , Jeremy G. Owen
- & David F. Ackerley
-
Article
| Open Access A role for annexin A2 in scaffolding the peroxiredoxin 2–STAT3 redox relay complex
A role for annexin A2 in scaffolding the peroxiredoxin 2–STAT3 redox relay complex
Peroxiredoxin 2 (Prx2) was previously shown to transfer H2O2-derived oxidative equivalents to STAT3, generating disulfide-linked dimers and tetramers. Here the authors show that the interaction between Prx2 and STAT3 at the plasma membrane is mediated by the membrane chaperone annexin A2; suggesting that the redox relay complex is part of a membrane signaling domain.
- Deepti Talwar
- , Joris Messens
- & Tobias P. Dick
-
Article
| Open Access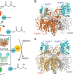 Gating mechanism of elongating β-ketoacyl-ACP synthases
Gating mechanism of elongating β-ketoacyl-ACP synthases
The formation of C-C bonds in fatty acid and polyketide biosynthesis depends on β-ketoacyl-acyl carrier protein (ACP) synthases (KSs). Here, the authors present structures of E.coli KSs bound to substrate mimetic bearing ACPs, providing insights into the catalytic mechanism underlying C-C bond forming reactions.
- Jeffrey T. Mindrebo
- , Ashay Patel
- & Michael D. Burkart
-
Article
| Open Access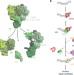 Single-molecule imaging reveals molecular coupling between transcription and DNA repair machinery in live cells
Single-molecule imaging reveals molecular coupling between transcription and DNA repair machinery in live cells
Mfd recognizes stalled transcriptional complexes at sites of lesions and recruits the nucleotide excision repair proteins (UvrAB) to the site. Here the authors use live cell imaging in E. coli to demonstrate that coordinated ATP hydrolysis by UvrA and loading of UvrB on DNA facilitate the dissociation of Mfd from the handoff complex.
- Han Ngoc Ho
- , Antoine M. van Oijen
- & Harshad Ghodke
-
Article
| Open Access Cryo-EM structure of the respiratory syncytial virus RNA polymerase
Cryo-EM structure of the respiratory syncytial virus RNA polymerase
Respiratory syncytial virus (RSV) is a pathogenic non-segmented negative-sense RNA virus and active RSV polymerase is composed of a 250 kDa large (L) protein and tetrameric phosphoprotein (P). Here, the authors present the 3.67 Å cryo-EM structure of the RSV polymerase (L:P) complex.
- Dongdong Cao
- , Yunrong Gao
- & Bo Liang
-
Article
| Open Access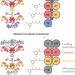 An in vitro platform for engineering and harnessing modular polyketide synthases
An in vitro platform for engineering and harnessing modular polyketide synthases
A robust platform to study modular polyketide synthases (PKSs) in vitro is still unavailable. Here, the authors report the reconstitution of the venemycin PKS, engineer hybrid venemycin/pikromycin PKSs, and obtain much improved yields through employing the updated module boundaries.
- Takeshi Miyazawa
- , Melissa Hirsch
- & Adrian T. Keatinge-Clay
-
Article
| Open Access Molecular basis for metabolite channeling in a ring opening enzyme of the phenylacetate degradation pathway
Molecular basis for metabolite channeling in a ring opening enzyme of the phenylacetate degradation pathway
The bacterial enzyme PaaZ is involved in the breakdown of environmental pollutants via the aerobic-anaerobic hybrid pathway but its substrate transfer mechanism is not fully understood. Here, the authors present cryoEM structures of free and ligand-bound PaaZ that suggest a mechanism for internal substrate channeling.
- Nitish Sathyanarayanan
- , Giuseppe Cannone
- & Kutti R. Vinothkumar
-
Article
| Open Access Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin
Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin
The mechanism of iron-sulfur (Fe-S) cluster biosynthesis is not fully understood. Here, the authors develop a physiologically relevant in vitro model of Fe-S cluster assembly, allowing them to elucidate the sequence of Fe-S cluster synthesis along with the respective roles of ferredoxin-2 and frataxin.
- Sylvain Gervason
- , Djabir Larkem
- & Benoit D’Autréaux
-
Article
| Open Access Reconstitution of recombinant human CCR4-NOT reveals molecular insights into regulated deadenylation
Reconstitution of recombinant human CCR4-NOT reveals molecular insights into regulated deadenylation
The CCR4-NOT complex shortens poly(A) tails of messenger RNAs. By biochemical reconstitution of the entire human CCR4-NOT complex, the authors show the stimulatory roles of non-enzymatic subunits and the importance of the interaction between CAF40 and RNA binding proteins in targeted deadenylation.
- Tobias Raisch
- , Chung-Te Chang
- & Eugene Valkov
-
Article
| Open Access Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus
Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus
The cell wall of Gram-positive bacteria consists of peptidoglycan modified with other polymers, such as the capsular polysaccharide. Here, the authors reconstitute the biosynthesis of capsular polysaccharide and elucidate its interplay with the cell wall biosynthetic machinery.
- Marvin Rausch
- , Julia P. Deisinger
- & Tanja Schneider
-
Article
| Open Access Pathogenic variants in glutamyl-tRNAGln amidotransferase subunits cause a lethal mitochondrial cardiomyopathy disorder
Pathogenic variants in glutamyl-tRNAGln amidotransferase subunits cause a lethal mitochondrial cardiomyopathy disorder
Mitochondrial protein synthesis requires charging a mitochondrial tRNA with its amino acid. Here, the authors describe pathogenic variants in the GatCAB protein complex genes required for the generation of glutaminyl-mt-tRNAGln, that impairs mitochondrial translation and presents with cardiomyopathy.
- Marisa W. Friederich
- , Sharita Timal
- & Johan L. K. Van Hove
-
Article
| Open Access Reprogramming of the antimycin NRPS-PKS assembly lines inspired by gene evolution
Reprogramming of the antimycin NRPS-PKS assembly lines inspired by gene evolution
Modifying the non-ribosomal peptide synthase (NRPS)/polyketide synthase (PKS) pathway to generate novel non-ribosomal peptides often results in a loss of productivity. Here the authors use evolutionary alignments of NRPS/PKS gene clusters to guide rational design of complexes that can produce novel lactones.
- Takayoshi Awakawa
- , Takuma Fujioka
- & Ikuro Abe
-
Article
| Open Access A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion
A promiscuous cytochrome P450 aromatic O-demethylase for lignin bioconversion
Catabolizing lignin-derived aromatic compounds requires an aryl-O-demethylation step. Here the authors present the structures of GcoA and GcoB, a cytochrome P450-reductase pair that catalyzes aryl-O-demethylations and show that GcoA displays broad substrate specificity, which is of interest for biotechnology applications.
- Sam J. B. Mallinson
- , Melodie M. Machovina
- & John E. McGeehan
-
Review Article
| Open Access The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation
The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation
Temporary association of metabolic enzymes is generally assumed to facilitate substrate channelling within the complex. In this review, Lee Sweetlove and Alisdair Fernie outline the nature and functional consequence of organising enzymes into assemblies, and discuss applications within the natural world and synthetic biology.
- Lee J. Sweetlove
- & Alisdair R. Fernie
-
Article
| Open Access Allosteric regulation alters carrier domain translocation in pyruvate carboxylase
Allosteric regulation alters carrier domain translocation in pyruvate carboxylase
Carrier domain enzymes accomplish catalysis by physically transporting intermediates long distances between remote active sites. Here the authors describe a wide range of catalytically productive translocation events during catalysis by pyruvate carboxylase and suggest a basis for its allosteric activation.
- Yumeng Liu
- , Melissa M. Budelier
- & Martin St. Maurice
-
Article
| Open Access Biotransformation of polyunsaturated fatty acids to bioactive hepoxilins and trioxilins by microbial enzymes
Biotransformation of polyunsaturated fatty acids to bioactive hepoxilins and trioxilins by microbial enzymes
Hepoxilins (HXs) and trioxilins (TrXs) are lipid metabolites with roles in inflammation and insulin secretion. Here, the authors discover a prokaryotic source of HXs and TrXs, identify the biosynthetic enzymes and heterologously express HXs and TrXs in E. coli.
- Jung-Ung An
- , Yong-Seok Song
- & Deok-Kun Oh
-
Article
| Open Access Structural analysis of mtEXO mitochondrial RNA degradosome reveals tight coupling of nuclease and helicase components
Structural analysis of mtEXO mitochondrial RNA degradosome reveals tight coupling of nuclease and helicase components
The mitochondrial RNA degradosome (mtEXO) plays an essential role in the regulation of mitochondrial gene expression and is composed of the 3′-to-5′ exoribonuclease Dss1 and the helicase Suv3. Here the authors present the RNA bound mtEXO crystal structure and give insights into its mechanism.
- Michal Razew
- , Zbigniew Warkocki
- & Marcin Nowotny
-
Article
| Open Access Targeting the CoREST complex with dual histone deacetylase and demethylase inhibitors
Targeting the CoREST complex with dual histone deacetylase and demethylase inhibitors
Alteration of the epigenetic landscape has been implicated in several disease processes, where targeting histone modifiers may have therapeutic applications. Here the authors report a bifunctional small molecule inhibitor that simultaneously targets the deacetylase (HDAC1) and demethylase (LSD1) activities of the CoREST complex.
- Jay H. Kalin
- , Muzhou Wu
- & Philip A. Cole
-
Article
| Open Access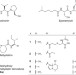 Warhead biosynthesis and the origin of structural diversity in hydroxamate metalloproteinase inhibitors
Warhead biosynthesis and the origin of structural diversity in hydroxamate metalloproteinase inhibitors
Metalloproteinase inhibitors are leads for drug development, but their biosynthetic pathways are often unknown. Here the authors show that the acyl branched warhead of actinonin and matlystatins derives from an ethylmalonyl-CoA-like pathway and the structural diversity of matlystatins is due to the activity of a decarboxylase-dehydrogenase enzyme.
- Franziska Leipoldt
- , Javier Santos-Aberturas
- & Leonard Kaysser
-
Article
| Open Access Decoding and reprogramming fungal iterative nonribosomal peptide synthetases
Decoding and reprogramming fungal iterative nonribosomal peptide synthetases
Nonribosomal peptides are important bioactive molecules that are synthetized by enzymes containing several catalytic domains. Here the authors describe the catalytic mechanism of fungal nonribosomal peptide synthetases and present an approach to modify these enzymes to produce specific nonribosomal peptides.
- Dayu Yu
- , Fuchao Xu
- & Jixun Zhan
-
Article
| Open Access Protein-protein interactions and metabolite channelling in the plant tricarboxylic acid cycle
Protein-protein interactions and metabolite channelling in the plant tricarboxylic acid cycle
A metabolon is a complex of sequential metabolic enzymes that channels substrates directly between enzymes, thus optimizing metabolic flux. Here Zhanget al. provide protein interaction and isotope dilution data that support the existence of a metabolon that channels both citrate and fumarate in the plant TCA cycle.
- Youjun Zhang
- , Katherine F. M. Beard
- & Toshihiro Obata
-
Article
| Open Access A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units
A crotonyl-CoA reductase-carboxylase independent pathway for assembly of unusual alkylmalonyl-CoA polyketide synthase extender units
Polyketides are typically assembled from a starter unit and malonyl- and/or methylmalonyl-CoA-derived extender units, but the macrolide antibiotics stambomycins incorporate non-standard alkylmalonyl-CoA extender units. Here, the authors describe the biosynthetic pathway responsible for this unusual synthesis.
- Lauren Ray
- , Timothy R. Valentic
- & Gregory L. Challis

 Resurrecting ancestral antibiotics: unveiling the origins of modern lipid II targeting glycopeptides
Resurrecting ancestral antibiotics: unveiling the origins of modern lipid II targeting glycopeptides