Featured
-
-
Article
| Open Access A hemoprotein with a zinc-mirror heme site ties heme availability to carbon metabolism in cyanobacteria
A hemoprotein with a zinc-mirror heme site ties heme availability to carbon metabolism in cyanobacteria
Heme is an abundant cofactor required by nearly all known organisms. Here, authors discover a cyanobacterial protein with a distinct Zn-mirror heme site, which may function to sense heme and regulate energy metabolism.
- Nicolas Grosjean
- , Estella F. Yee
- & Crysten E. Blaby-Haas
-
Article
| Open Access Widespread extracellular electron transfer pathways for charging microbial cytochrome OmcS nanowires via periplasmic cytochromes PpcABCDE
Widespread extracellular electron transfer pathways for charging microbial cytochrome OmcS nanowires via periplasmic cytochromes PpcABCDE
How do cells put electrons to rest? Using a minimal pathway to get rid of excess metabolic electrons, diverse environmentally important microbes overcome large spatial, kinetic, and thermodynamic barriers in order to survive in extreme anoxic conditions.
- Pilar C. Portela
- , Catharine C. Shipps
- & Nikhil S. Malvankar
-
Article
| Open Access A natural biogenic nanozyme for scavenging superoxide radicals
A natural biogenic nanozyme for scavenging superoxide radicals
The inorganic minerals are believed to exert a critical catalytic role in the prebiotic time, but biominerals (e.g., bones) in modern living organisms are known mainly for their physical property-related functions. Here the authors identify natural ferritin iron core as a superoxide dismutase-like nanozyme exhibiting species-related activity and elucidate its specific catalytic mechanism.
- Long Ma
- , Jia-Jia Zheng
- & Kelong Fan
-
Article
| Open Access Human apical-out nasal organoids reveal an essential role of matrix metalloproteinases in airway epithelial differentiation
Human apical-out nasal organoids reveal an essential role of matrix metalloproteinases in airway epithelial differentiation
Airway organoids made in Matrigel are in basal-out orientation. Here, authors present apical-out nasal organoids using a biochemically defined hydrogel system, revealing that matrix metalloproteinases are required for normal epithelial development.
- Liyue Li
- , Linyi Jiao
- & Chunwei Li
-
Article
| Open Access Structure of full-length cobalamin-dependent methionine synthase and cofactor loading captured in crystallo
Structure of full-length cobalamin-dependent methionine synthase and cofactor loading captured in crystallo
Methionine synthase (MS) harnesses B12 and flexibility to catalyze three different reactions on one protein. The full-length structure of MS yields insights into a protein that epitomizes controlled dynamics to dictate chemical outcome.
- Johnny Mendoza
- , Meredith Purchal
- & Markos Koutmos
-
Article
| Open Access TerC proteins function during protein secretion to metalate exoenzymes
TerC proteins function during protein secretion to metalate exoenzymes
TerC family membrane proteins associate with the general protein secretion complex to facilitate the co-translocational loading of Mn(II) into nascent metalloenzymes. Here, the authors show that Bacillus subtilis mutants lacking TerC proteins are defective in production of the membrane-embedded lipoteichoic acid synthase and secreted proteases.
- Bixi He
- , Ankita J. Sachla
- & John D. Helmann
-
Article
| Open Access Custom tuning of Rieske oxygenase reactivity
Custom tuning of Rieske oxygenase reactivity
Rieske oxygenase chemistry is important for biochemical pathways, but it remains elusive how a common protein scaffold can be predictively tuned to catalyze divergent reactions. Here, the authors report a strategy that can rationally tune TsaM, a Rieske monooxygenase to catalyze dioxygenation and sequential monooxygenation reactions, and customize the reactivity of other Rieske oxygenases.
- Jiayi Tian
- , Jianxin Liu
- & Jennifer Bridwell-Rabb
-
Article
| Open Access Class III hybrid cluster protein homodimeric architecture shows evolutionary relationship with Ni, Fe-carbon monoxide dehydrogenases
Class III hybrid cluster protein homodimeric architecture shows evolutionary relationship with Ni, Fe-carbon monoxide dehydrogenases
Here, the authors present an X-ray crystal structure of a class III hybrid cluster protein (HCP), structurally similar to Ni, Fe-carbon monoxide dehydrogenases (CODHs), enabling conclusions to be drawn regarding the structural evolution of HCP/CODH superfamily.
- Takashi Fujishiro
- & Kyosei Takaoka
-
Article
| Open Access Architecture of the Heme-translocating CcmABCD/E complex required for Cytochrome c maturation
Architecture of the Heme-translocating CcmABCD/E complex required for Cytochrome c maturation
The covalent linkage of hemes to cytochromes c requires a maturation machinery. Here, the authors provide mechanistic insights into how the heme translocase complex CcmABCD flops a heme group, driven by ATP hydrolysis, and delivers it to the chaperone CcmE.
- Lorena Ilcu
- , Lukas Denkhaus
- & Oliver Einsle
-
Article
| Open Access The Ycf48 accessory factor occupies the site of the oxygen-evolving manganese cluster during photosystem II biogenesis
The Ycf48 accessory factor occupies the site of the oxygen-evolving manganese cluster during photosystem II biogenesis
The Ycf48 accessory factor is important for the assembly and repair of the photosystem II (PSII) complex of oxygenic photosynthesis. Here, the authors show that Ycf48 occupies the binding site of the oxygen evolving Mn cluster early in PSII biogenesis.
- Ziyu Zhao
- , Irene Vercellino
- & Josef Komenda
-
Article
| Open Access A 2.2 Å cryoEM structure of a quinol-dependent NO Reductase shows close similarity to respiratory oxidases
A 2.2 Å cryoEM structure of a quinol-dependent NO Reductase shows close similarity to respiratory oxidases
Quinol-dependent nitric oxide reductases, unique to bacteria, are considered members of respiratory heme copper oxidases. A 2.2 Å cryoEM structure of qNOR is reported shedding light on key aspects of enzyme mechanism including quinol binding and pathways for electron, substrate, and proton transport.
- Alex J. Flynn
- , Svetlana V. Antonyuk
- & S. Samar Hasnain
-
Article
| Open Access Metal3D: a general deep learning framework for accurate metal ion location prediction in proteins
Metal3D: a general deep learning framework for accurate metal ion location prediction in proteins
Zinc is an essential metal for many proteins. Here, the authors propose a model based on 3D convolutional networks to predict the location of zinc in experimental and computationally predicted structures within a framework readily extensible to other metals.
- Simon L. Dürr
- , Andrea Levy
- & Ursula Rothlisberger
-
Article
| Open Access Designed Rubredoxin miniature in a fully artificial electron chain triggered by visible light
Designed Rubredoxin miniature in a fully artificial electron chain triggered by visible light
Living organisms regulate their energy demand by managing electron trafficking in complex transport chains. Here, the authors pioneer a fully artificial electron chain triggered by visible light using designed proteins, unlocking possibilities in bioengineering.
- Marco Chino
- , Luigi Franklin Di Costanzo
- & Vincenzo Pavone
-
Article
| Open Access Structural consequences of turnover-induced homocitrate loss in nitrogenase
Structural consequences of turnover-induced homocitrate loss in nitrogenase
Biological nitrogen fixation is achieved by nitrogenase, but the mechanism remains enigmatic. Here, the authors report high resolution single particle cryoEM structures of homocitrate-compromised MoFe-proteins and unveil a new binding partner.
- Rebeccah A. Warmack
- , Ailiena O. Maggiolo
- & Douglas C. Rees
-
Article
| Open Access Characterization of paramagnetic states in an organometallic nickel hydrogen evolution electrocatalyst
Characterization of paramagnetic states in an organometallic nickel hydrogen evolution electrocatalyst
The characterization of nickel (Ni)‐centred paramagnetic states relevant to [NiFe] hydrogenases is rare in mononuclear Ni hydrogen evolution catalysts. Here, the authors report the spectroscopic and synthetic characterization of NiI and NiIII states in an organometallic Ni hydrogen evolution catalyst.
- Sagnik Chakrabarti
- , Soumalya Sinha
- & Liviu M. Mirica
-
Article
| Open Access Iron-sulfur clusters are involved in post-translational arginylation
Iron-sulfur clusters are involved in post-translational arginylation
The enzyme ATE1 catalyzes eukaryotic post-translation arginylation, a key protein modification necessary for cellular homeostasis. Here, the authors show that ATE1s are previously unrealized iron-sulfur proteins that use this oxygen-sensitive inorganic cofactor to control cellular arginylation
- Verna Van
- , Janae B. Brown
- & Aaron T. Smith
-
Article
| Open Access Ru(II) photocages enable precise control over enzyme activity with red light
Ru(II) photocages enable precise control over enzyme activity with red light
The cytochrome P450 enzyme CYP1B1 is overexpressed in a variety of tumors, and is correlated with poor treatment outcomes; thus, it is desirable to develop CYP1B1 inhibitors to restore chemotherapy efficacy. Here the authors describe the creation of light-triggered CYP1B1 inhibitors as “prodrugs”, and achieve >6000-fold improvement in potency upon activation with low-energy (660 nm) light.
- Dmytro Havrylyuk
- , Austin C. Hachey
- & Edith C. Glazer
-
Article
| Open Access Metal-responsive regulation of enzyme catalysis using genetically encoded chemical switches
Metal-responsive regulation of enzyme catalysis using genetically encoded chemical switches
Dynamic control over protein function is a central challenge in synthetic biology. Here the authors present an integrated computational and experimental workflow for engineering reversible protein switches; metal-chelating unnatural amino acids genetically encoded into two conformationally dynamic enzymes to yield robust switches.
- Yasmine S. Zubi
- , Kosuke Seki
- & Jared C. Lewis
-
Article
| Open Access Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression
Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression
Considering the intrinsic toxicities of transition metals, their incorporation into drug therapies must operate at minimal amounts while ensuring adequate catalytic activity within complex biological systems. This study investigates the design of synthetic prodrugs that not only can be tuned to be harmless, but are robustly transformed in vivo to reach therapeutically relevant levels.
- Igor Nasibullin
- , Ivan Smirnov
- & Katsunori Tanaka
-
Article
| Open Access Protein interface redesign facilitates the transformation of nanocage building blocks to 1D and 2D nanomaterials
Protein interface redesign facilitates the transformation of nanocage building blocks to 1D and 2D nanomaterials
Various strategies to assemble protein building blocks into one-, two- and three-dimensional hierarchical nanostructures were described, but controlling the transformation between those different assemblies is largely uninvestigated. Here, the authors describe a protein interface redesign strategy and use it for the self-assembly transformation of dimeric building blocks from hollow protein nanocage to filament, nanorod and nanoribbon.
- Xiaorong Zhang
- , Yu Liu
- & Guanghua Zhao
-
Article
| Open Access Heme biosynthesis depends on previously unrecognized acquisition of iron-sulfur cofactors in human amino-levulinic acid dehydratase
Heme biosynthesis depends on previously unrecognized acquisition of iron-sulfur cofactors in human amino-levulinic acid dehydratase
Heme biosynthesis depends on iron-sulfur (Fe-S) cluster biogenesis but the molecular connection between these pathways is not fully understood. Here, the authors show that the heme biosynthesis enzyme ALAD contains an Fe-S cluster, disruption of which reduces ALAD activity and heme production in human cells.
- Gang Liu
- , Debangsu Sil
- & Tracey Ann Rouault
-
Article
| Open Access Structure of the respiratory MBS complex reveals iron-sulfur cluster catalyzed sulfane sulfur reduction in ancient life
Structure of the respiratory MBS complex reveals iron-sulfur cluster catalyzed sulfane sulfur reduction in ancient life
The sulfur-reducing enzyme MBS and the hydrogen-gas evolving MBH are the evolutionary link between the ancestor Mrp antiporter and the mitochondrial respiratory complex I. Here, the authors characterise MBS from the hyperthermophilic archaeon Pyrococcus furiosus, solve its cryo-EM structure and discuss the structural evolution from Mrp to MBH and MBS and to the modern-day complex I.
- Hongjun Yu
- , Dominik K. Haja
- & Michael W. W. Adams
-
Article
| Open Access Elucidating the role of metal ions in carbonic anhydrase catalysis
Elucidating the role of metal ions in carbonic anhydrase catalysis
Metalloenzymes often show different catalytic activities in the presence of non-native metal ions. Here, the authors report structures of carbonic anhydrase bound to zinc and several other metal ions and demonstrate that metal ion coordination geometries directly impact catalytic activity of the enzyme.
- Jin Kyun Kim
- , Cheol Lee
- & Chae Un Kim
-
Article
| Open Access An evolutionary path to altered cofactor specificity in a metalloenzyme
An evolutionary path to altered cofactor specificity in a metalloenzyme
Many metalloenzymes are highly specific for their cognate metal ion but the molecular principles underlying this specificity often remain unclear. Here, the authors characterize the structural and biochemical basis for the different metal specificity of two evolutionarily related superoxide dismutases.
- Anna Barwinska-Sendra
- , Yuritzi M. Garcia
- & Kevin J. Waldron
-
Article
| Open Access Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy
Assessing electron transfer reactions and catalysis in multicopper oxidases with operando X-ray absorption spectroscopy
Understanding enzyme active sites can elucidate fundamental enzymatic reaction pathways and inform designs for synthetic catalysts. Here, authors employ operando X-ray absorption spectroelectrochemistry to assess copper ions in bilirubin oxidase during oxygen reduction electrocatalysis.
- Lucyano J. A. Macedo
- , Ayaz Hassan
- & Frank N. Crespilho
-
Article
| Open Access Heme and hemoglobin utilization by Mycobacterium tuberculosis
Heme and hemoglobin utilization by Mycobacterium tuberculosis
Iron is essential for growth of Mycobacterium tuberculosis, but most of the iron in the human body is stored in heme within hemoglobin. Here, Mitra et al. identify two heme uptake mechanisms in M. tuberculosis, one dependent on the inner-membrane Dpp importer and the other dependent on host albumin.
- Avishek Mitra
- , Ying-Hui Ko
- & Michael Niederweis
-
Article
| Open Access Protein determinants of dissemination and host specificity of metallo-β-lactamases
Protein determinants of dissemination and host specificity of metallo-β-lactamases
Metallo-β-lactamases (MBLs) confer resistance to carbapenem antibiotics. Here, López et al. show that the host range of MBLs depends on the efficiency of MBL signal peptide processing and secretion into outer membrane vesicles, which affects bacterial fitness.
- Carolina López
- , Juan A. Ayala
- & Alejandro J. Vila
-
Article
| Open Access Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin
Physiologically relevant reconstitution of iron-sulfur cluster biosynthesis uncovers persulfide-processing functions of ferredoxin-2 and frataxin
The mechanism of iron-sulfur (Fe-S) cluster biosynthesis is not fully understood. Here, the authors develop a physiologically relevant in vitro model of Fe-S cluster assembly, allowing them to elucidate the sequence of Fe-S cluster synthesis along with the respective roles of ferredoxin-2 and frataxin.
- Sylvain Gervason
- , Djabir Larkem
- & Benoit D’Autréaux
-
Article
| Open Access Native top-down mass spectrometry provides insights into the copper centers of membrane-bound methane monooxygenase
Native top-down mass spectrometry provides insights into the copper centers of membrane-bound methane monooxygenase
The activity of the membrane-bound enzyme pMMO depends on copper but the location of the copper centers is still under debate. Here, the authors reconstitute pMMO in nanodiscs and use native top-down MS to localize its copper centers, providing insights into which sites are essential for activity.
- Soo Y. Ro
- , Luis F. Schachner
- & Amy C. Rosenzweig
-
Article
| Open Access Proton mediated spin state transition of cobalt heme analogs
Proton mediated spin state transition of cobalt heme analogs
Studying the electronic structures and spin transitions of synthetic heme analogs is crucial to advancing our understanding of heme enzyme mechanisms. Here the authors show that a Co(II) porphyrin complex undergoes an unexpected spin state transition upon deprotonation of its axial imidazole ligand.
- Jianping Zhao
- , Qian Peng
- & Jianfeng Li
-
Article
| Open Access Cryo-EM structure of the human ferritin–transferrin receptor 1 complex
Cryo-EM structure of the human ferritin–transferrin receptor 1 complex
The human transferrin receptor 1 (CD71) is a transmembrane protein responsible for iron uptake. Here the authors present the 3.9 Å resolution cryo-EM structure of the CD71 ectodomain-human ferritin (H-Ft) complex and find that H-Ft binds a CD71 region different from the transferrin one that overlaps with the surface recognized by select pathogens.
- Linda Celeste Montemiglio
- , Claudia Testi
- & Beatrice Vallone
-
Article
| Open Access A widely distributed diheme enzyme from Burkholderia that displays an atypically stable bis-Fe(IV) state
A widely distributed diheme enzyme from Burkholderia that displays an atypically stable bis-Fe(IV) state
The diheme enzyme MauG forms a bis-Fe(IV) state. Here the authors identify and determine the structure of BthA, a diheme peroxidase conserved in all Burkholderia and show that BthA also forms a bis-Fe(IV) species but mechanistically differs from MauG by combining magnetic resonance, near-IR and Mössbauer spectroscopies and electrochemical methods.
- Kimberly Rizzolo
- , Steven E. Cohen
- & Sean J. Elliott
-
Article
| Open Access Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein
Tetrathiomolybdate induces dimerization of the metal-binding domain of ATPase and inhibits platination of the protein
Tetrathiomolybdate (TM) and Cu-ATPases, e.g. Wilson (WLN) protein, affect the efficacy of common anticancer drug cisplatin. Here, the authors show that TM generates a protein dimer with a WLN domain by expelling copper and provide insight into the synergy of TM and cisplatin in cancer chemotherapy.
- Tiantian Fang
- , Wanbiao Chen
- & Yangzhong Liu
-
Article
| Open Access Hemocyanin facilitates lignocellulose digestion by wood-boring marine crustaceans
Hemocyanin facilitates lignocellulose digestion by wood-boring marine crustaceans
Marine woodborers can digest woody biomass without the help of gut microbiota but the mechanism has remained unclear. Here, the authors provide evidence that the woodborer’s respiratory protein hemocyanin plays a central role in wood digestion and may offer a route toward biorefining of woody plant biomass.
- Katrin Besser
- , Graham P. Malyon
- & Simon J. McQueen-Mason
-
Article
| Open Access Ultrafast carbon monoxide photolysis and heme spin-crossover in myoglobin via nonadiabatic quantum dynamics
Ultrafast carbon monoxide photolysis and heme spin-crossover in myoglobin via nonadiabatic quantum dynamics
Myoglobin bound to carbon monoxide undergoes an ultrafast light-induced reaction, which ends up in a photolyzed carbon monoxide and a spin transition of the iron center. Here, the authors employ quantum wavepacket dynamics to show that photolysis precedes the spin transition, a mechanism dominated by strong electron-nuclear couplings.
- Konstantin Falahati
- , Hiroyuki Tamura
- & Miquel Huix-Rotllant
-
Article
| Open Access Characterization of a long overlooked copper protein from methane- and ammonia-oxidizing bacteria
Characterization of a long overlooked copper protein from methane- and ammonia-oxidizing bacteria
Methane- and ammonia-oxidizing bacteria use the integral membrane, copper-dependent enzymes particulate methane monooxygenase (pMMO) and ammonia monooxygenase (AMO) to oxidize methane and ammonia. Here the authors structurally characterize the copper-binding protein PmoD, which contains an unusual CuA site and their genetic analyses strongly support a pMMO and AMO related function of PmoD.
- Oriana S. Fisher
- , Grace E. Kenney
- & Amy C. Rosenzweig
-
Article
| Open Access Control of transmembrane charge transfer in cytochrome c oxidase by the membrane potential
Control of transmembrane charge transfer in cytochrome c oxidase by the membrane potential
Cytochrome c oxidase (CytcO) is the last enzyme of the electron transport chain, but how the electrochemical membrane potential affects CytcO is unclear. Here the authors show that proton uptake to the catalytic site of CytcO and presumably proton translocation was impaired by the potential, but electron transfer was not affected.
- Markus L. Björck
- & Peter Brzezinski
-
Article
| Open Access Probing the coordination and function of Fe4S4 modules in nitrogenase assembly protein NifB
Probing the coordination and function of Fe4S4 modules in nitrogenase assembly protein NifB
NifB is a key enzyme in the biosynthesis pathway of the nitrogenase FeMo cofactor. Here, the authors investigate the maturation of its iron-sulfur clusters by EPR and biochemical analyses, showing how individual precursor clusters participate in the formation of the final iron-sulfur cluster.
- Lee A. Rettberg
- , Jarett Wilcoxen
- & Yilin Hu
-
Article
| Open Access Bacterial encapsulins as orthogonal compartments for mammalian cell engineering
Bacterial encapsulins as orthogonal compartments for mammalian cell engineering
Artificial compartments have been expressed in prokaryotes and yeast, but similar capabilities have been missing for mammalian cell engineering. Here the authors use bacterial encapsulins to engineer genetically controlled multifunctional orthogonal compartments in mammalian cells.
- Felix Sigmund
- , Christoph Massner
- & Gil G. Westmeyer
-
Article
| Open Access A mechanism for CO regulation of ion channels
A mechanism for CO regulation of ion channels
Despite being highly toxic, carbon monoxide (CO) is also essential as an intracellular signalling molecule, but CO-dependent signalling is poorly understood. Here, authors employ spectroscopic and electrophysiology methods and find that CO activates KATP channels via SUR2A, a heme-regulated receptor.
- Sofia M. Kapetanaki
- , Mark J. Burton
- & Emma Raven
-
Article
| Open Access Structure of a Wbl protein and implications for NO sensing by M. tuberculosis
Structure of a Wbl protein and implications for NO sensing by M. tuberculosis
Mycobacterium tuberculosis WhiB1 is a DNA-binding protein with a NO sensitive [4Fe-4S] cluster. Here the authors present the NMR structure of WhiB1 and suggest how loss of the iron-sulfur cluster through nitrosylation affects WhiB1 DNA binding and leads to transcriptional reprogramming.
- Bassam K. Kudhair
- , Andrea M. Hounslow
- & Jeffrey Green
-
Article
| Open Access Fine control of metal concentrations is necessary for cells to discern zinc from cobalt
Fine control of metal concentrations is necessary for cells to discern zinc from cobalt
Bacteria possess transcription factors whose DNA-binding activity is altered upon binding to specific metals, but the binding of metals is not specific in vitro. Here, Osman et al. show that tight regulation of buffered intracellular metal concentrations is a prerequisite for metal specificity.
- Deenah Osman
- , Andrew W. Foster
- & Nigel J. Robinson
-
Article
| Open Access Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx
Biogenic manganese oxide nanoparticle formation by a multimeric multicopper oxidase Mnx
Significant challenges exist for structural characterization of enzymes responsible for biomineralization. Here the authors show that native mass spectrometry and high resolution electron microscopy can define the subunit topology and copper binding of a manganese oxidizing complex, and describe early stage formation of its mineral products
- Christine A. Romano
- , Mowei Zhou
- & Bradley M. Tebo
-
Article
| Open Access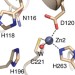 A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases
A general reaction mechanism for carbapenem hydrolysis by mononuclear and binuclear metallo-β-lactamases
Carbapenem-resistant bacteria pose a major health threat by expressing metallo-β-lactamases (MβLs), enzymes able to hydrolyse these life-saving drugs. Here the authors use biophysical and computational methods and show that different MβLs share the same reaction mechanism, suggesting new strategies for drug design.
- María-Natalia Lisa
- , Antonela R. Palacios
- & Alejandro J. Vila
-
Article
| Open Access Biological iron-sulfur storage in a thioferrate-protein nanoparticle
Biological iron-sulfur storage in a thioferrate-protein nanoparticle
The biosynthesis of iron-sulfur clusters in anaerobic organisms has not been extensively investigated. Here, the authors identify and characterize a multi-subunit protein that stores iron and sulfur in thioferrate for the assembly of the clusters inPyrococcus furiosus.
- Brian J. Vaccaro
- , Sonya M. Clarkson
- & Michael W. W. Adams
-
Article
| Open Access ISCA1 is essential for mitochondrial Fe4S4 biogenesis in vivo
ISCA1 is essential for mitochondrial Fe4S4 biogenesis in vivo
The mitochondrial proteins ISCA1 and ISCA2 form a complex that is involved in the biogenesis of Fe–S clusters. Here the authors report that ISCA1 and ISCA2 interact differently with proteins of the Fe–S machinery and that under certain conditions, ISCA2 seems dispensable for Fe–S biogenesis.
- Lena Kristina Beilschmidt
- , Sandrine Ollagnier de Choudens
- & Alain Martelli
-
Article
| Open Access Observation of gold sub-nanocluster nucleation within a crystalline protein cage
Observation of gold sub-nanocluster nucleation within a crystalline protein cage
Proteins can template the synthesis of inorganic nanoparticles, but the formation mechanisms remain vague. Here, the authors directly observe, through a sequence of X-ray crystal structures, the stages of gold sub-nanocluster growth within the confined environment of a ferritin cage.
- Basudev Maity
- , Satoshi Abe
- & Takafumi Ueno
-
Article
| Open Access The in vivo hydrocarbon formation by vanadium nitrogenase follows a secondary metabolic pathway
The in vivo hydrocarbon formation by vanadium nitrogenase follows a secondary metabolic pathway
Nitrogenases reduce inorganic nitrogen to organic ammonia in a crucial step of the nitrogen cycle. Here the authors show that the vanadium-nitrogenase ofAzotobacter vinelandii can also catalyse the in vivoconversion of carbon monoxide to hydrocarbons in a secondary non-biosynthetic pathway.
- Johannes G. Rebelein
- , Chi Chung Lee
- & Markus W. Ribbe
-
Article
| Open Access Sulfheme formation during homocysteine S-oxygenation by catalase in cancers and neurodegenerative diseases
Sulfheme formation during homocysteine S-oxygenation by catalase in cancers and neurodegenerative diseases
High levels of homocysteine in cells are linked to pathological states. Here, the authors report that homocysteine inactivates catalase by modifying the heme group, impairing cellular redox homeostasis, and show that this modification occurs in cancer cells and in a cellular model of Parkinson’s disease.
- Dominique Padovani
- , Assia Hessani
- & Isabelle Artaud

 Mechanism and structural dynamics of sulfur transfer during de novo [2Fe-2S] cluster assembly on ISCU2
Mechanism and structural dynamics of sulfur transfer during de novo [2Fe-2S] cluster assembly on ISCU2