Article
|
Open Access
Featured
-
-
Article
| Open Access Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP
Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP
The complete biosynthetic pathway from most natural products (NPs) are unknown. Here, the authors report BioNavi-NP, a computational toolkit for bio-retrosynthetic pathway elucidation or reconstruction for both NPs and NP-like compounds.
- Shuangjia Zheng
- , Tao Zeng
- & Ruibo Wu
-
Article
| Open Access Artificially sporulated Escherichia coli cells as a robust cell factory for interfacial biocatalysis
Artificially sporulated Escherichia coli cells as a robust cell factory for interfacial biocatalysis
Whole-cell biocatalysis is of interest for the efficient production of a range of products but cell stability can be an issue. Here, the authors turn recombinant enzyme-producing E. coli into artificial spores to add protection from UV radiation, heating, and organic solvents by coating the cells with polydopamine to create robust cell factories.
- Zhiyong Sun
- , René Hübner
- & Changzhu Wu
-
Article
| Open Access Extensible and self-recoverable proteinaceous materials derived from scallop byssal thread
Extensible and self-recoverable proteinaceous materials derived from scallop byssal thread
Bio-inspired materials are an intense area of study as researchers try to adapt biomaterials for other applications. Here, the authors report on the processing of protein materials derived from the byssal thread of scallops to create high-extensibility materials with self-recovery under wet conditions.
- Xiaokang Zhang
- , Mengkui Cui
- & Weizhi Liu
-
Article
| Open Access Discovery and characterization of a terpene biosynthetic pathway featuring a norbornene-forming Diels-Alderase
Discovery and characterization of a terpene biosynthetic pathway featuring a norbornene-forming Diels-Alderase
Pericyclase enzymes are an expanding family of enzymes. Here, the authors identify the norbornene synthase SdnG, a pericyclase for the intramolecular Diels-Alder reaction between a cyclopentadiene and an olefinic dienophile to form the sordaricin norbornene structure, and reconstitute the sordaricin biosynthesis.
- Zuodong Sun
- , Cooper S. Jamieson
- & Yi Tang
-
Article
| Open Access Correlational networking guides the discovery of unclustered lanthipeptide protease-encoding genes
Correlational networking guides the discovery of unclustered lanthipeptide protease-encoding genes
Many natural product biosynthetic gene clusters are found not to harbor a full set of necessary genes and must utilize enzymes encoded elsewhere (hidden) in the genome for biosynthesis. Here, the authors establish a global correlation network bridging the gap between lanthipeptide precursors and hidden proteases.
- Dan Xue
- , Ethan A. Older
- & Jie Li
-
Article
| Open Access A peroxisomal heterodimeric enzyme is involved in benzaldehyde synthesis in plants
A peroxisomal heterodimeric enzyme is involved in benzaldehyde synthesis in plants
Benzaldehyde is a simple aromatic aldehyde that attracts pollinators, has antifungal properties and contributes to flavor in many plants. Here the authors show that benzaldehyde is synthesized in petunia via the benzoic acid β-oxidative pathway by a peroxisomal heterodimeric enzyme consisting of α and β subunits.
- Xing-Qi Huang
- , Renqiuguo Li
- & Natalia Dudareva
-
Article
| Open Access Reconstituting the complete biosynthesis of D-lysergic acid in yeast
Reconstituting the complete biosynthesis of D-lysergic acid in yeast
The ergot alkaloids are a class of natural products known for their pharmacologically privileged molecular structure that are used in the treatment of neurological ailments. Here the authors report on the production of the ergot (fungus)-derived therapeutic precursor, D-lysergic acid (DLA), in baker’s yeast.
- Garrett Wong
- , Li Rong Lim
- & Wen Shan Yew
-
Article
| Open Access Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins
Engineering the stambomycin modular polyketide synthase yields 37-membered mini-stambomycins
Genetic engineering of the type I polyketide synthases (PKSs) to produce desirable analogous remains largely inefficient. Here, the authors leverage multiple approaches to delete seven internal modules from the stambomycin PKS and generate 37-membered mini-stambomycin macrolactones.
- Li Su
- , Laurence Hôtel
- & Kira J. Weissman
-
Article
| Open Access Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood
Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood
2-(2-Phenylethyl)chromones (PECs) contribute to the distinctive fragrance of agarwood. Here the authors identify a diarylpentanoid-producing polyketide synthase from Aquilaria sinensis and show how it catalyzes PEC formation.
- Xiao-Hui Wang
- , Bo-Wen Gao
- & She-Po Shi
-
Article
| Open Access Berberine bridge enzyme-like oxidase-catalysed double bond isomerization acts as the pathway switch in cytochalasin synthesis
Berberine bridge enzyme-like oxidase-catalysed double bond isomerization acts as the pathway switch in cytochalasin synthesis
Cytochalasans are a large family of fungal polyketide-nonribosomal peptide hybrid natural products that exhibit important pharmaceutical activities, but the mechanism of conversion of the monocytochalasans to the polycyclic, fused analogues is unclear. Here the authors reconstitute the core backbone of the cytochalasin family and describe an oxidase that catalyzes an unusual double-bond isomerization reaction.
- Jin-Mei Zhang
- , Xuan Liu
- & Yi Zou
-
Article
| Open Access Aminoacyl chain translocation catalysed by a type II thioesterase domain in an unusual non-ribosomal peptide synthetase
Aminoacyl chain translocation catalysed by a type II thioesterase domain in an unusual non-ribosomal peptide synthetase
Non-Ribosomal Peptide Synthetases (NRPSs) are responsible for the construction of many types of natural products. Here the authors characterize a key type II thioesterase domain that sheds light on the chain translocation processes of legonmycin NRPSs.
- Shan Wang
- , William D. G. Brittain
- & Hai Deng
-
Article
| Open Access Molecular insights into the unusually promiscuous and catalytically versatile Fe(II)/α-ketoglutarate-dependent oxygenase SptF
Molecular insights into the unusually promiscuous and catalytically versatile Fe(II)/α-ketoglutarate-dependent oxygenase SptF
Non-heme iron and α-ketoglutarate-dependent (Fe/αKG) oxygenases have attracted attention for their application as biocatalysts due to their flexibility and high efficiency. Here, the authors show the biochemical and structural characterizations of the versatile Fe/αKG oxygenase SptF, involved in the biosynthesis of fungal meroterpenoid emervaridones.
- Hui Tao
- , Takahiro Mori
- & Ikuro Abe
-
Article
| Open Access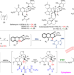 Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics
Reductive inactivation of the hemiaminal pharmacophore for resistance against tetrahydroisoquinoline antibiotics
Antibiotic-producing organisms need to co-evolve self-protection mechanisms to avoid any damage to themselves caused by the antibiotic pharmacophore (the reactive part of the compound). In this study, the authors report a self-defense strategy in naphthyridinomycin (NDM)-producing Streptomyces lusitanus, that comprises reductive inactivation of the hemiaminal pharmacophore by short-chain dehydrogenases/reductases (SDRs) NapW and HomW.
- Wan-Hong Wen
- , Yue Zhang
- & Gong-Li Tang
-
Article
| Open Access Gene editing enables rapid engineering of complex antibiotic assembly lines
Gene editing enables rapid engineering of complex antibiotic assembly lines
Engineering biosynthetic assembly lines is a powerful path to new natural products but is challenging with current methods. Here the authors use CRISPR-Cas9 to exchange subdomains within NRPS to alter substrate selectivity.
- Wei Li Thong
- , Yingxin Zhang
- & Jason Micklefield
-
Article
| Open Access Directed evolution of rRNA improves translation kinetics and recombinant protein yield
Directed evolution of rRNA improves translation kinetics and recombinant protein yield
Ribosome kinetics are rate-limiting for protein synthesis. Here the authors evolve diverse 16S rRNAs for enhanced protein synthesis rates and genetic code expansion efficiencies in vivo.
- Fan Liu
- , Siniša Bratulić
- & Ahmed H. Badran
-
Article
| Open Access Conformational rearrangements enable iterative backbone N-methylation in RiPP biosynthesis
Conformational rearrangements enable iterative backbone N-methylation in RiPP biosynthesis
Borosins are ribosomally encoded and posttranslationally modified peptide (RiPP) natural products featuring amide-backbone α-N-methylation. Here, the authors report the discovery and characterization of type IV borosin ‘split’ pathways encoding distinct, separate α-N-methyltransferases and precursor peptide substrates.
- Fredarla S. Miller
- , Kathryn K. Crone
- & Michael F. Freeman
-
Article
| Open Access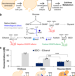 An integrated in vivo/in vitro framework to enhance cell-free biosynthesis with metabolically rewired yeast extracts
An integrated in vivo/in vitro framework to enhance cell-free biosynthesis with metabolically rewired yeast extracts
Cell-free systems enable the design of biosynthetic pathways for sustainable chemical synthesis. Here the authors create an integrated framework for accelerating design using extracts from genetically rewired strains.
- Blake J. Rasor
- , Xiunan Yi
- & Michael C. Jewett
-
Article
| Open Access Correcting for sparsity and interdependence in glycomics by accounting for glycan biosynthesis
Correcting for sparsity and interdependence in glycomics by accounting for glycan biosynthesis
Glycomics can uncover important molecular changes but measured glycans are highly interconnected and incompatible with common statistical methods, introducing pitfalls during analysis. Here, the authors develop an approach to identify glycan dependencies across samples to facilitate comparative glycomics.
- Bokan Bao
- , Benjamin P. Kellman
- & Nathan E. Lewis
-
Article
| Open Access Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan
Mapping the biosynthetic pathway of a hybrid polyketide-nonribosomal peptide in a metazoan
The only known animal polyketide-nonribosomal peptides, the nemamides, are biosynthesized by two megasynthetases in the canal-associated neurons (CANs) of C. elegans. Here, the authors map the biosynthetic roles of individual megasynthetase domains and identify additional enzymes in the CANs required for nemamide biosynthesis.
- Likui Feng
- , Matthew T. Gordon
- & Rebecca A. Butcher
-
Article
| Open Access Initiating polyketide biosynthesis by on-line methyl esterification
Initiating polyketide biosynthesis by on-line methyl esterification
Aurantinins are polyketides with unusual connectivities and broad antibacterial activity. Here the authors show the biosynthesis of aurantinins, which proceeds via an on-line methyl esterification at the terminus that enables the iterative chain elongations prior to condensation and cyclization.
- Pengwei Li
- , Meng Chen
- & Yihua Chen
-
Review Article
| Open Access Mining and unearthing hidden biosynthetic potential
Mining and unearthing hidden biosynthetic potential
Natural products are an important source of bioactive compounds and have versatile applications in different fields, but their discovery is challenging. Here, the authors review the recent developments in genome mining for discovery of natural products, focusing on compounds from unconventional microorganisms and microbiomes.
- Kirstin Scherlach
- & Christian Hertweck
-
Article
| Open Access Structural insight on assembly-line catalysis in terpene biosynthesis
Structural insight on assembly-line catalysis in terpene biosynthesis
Substrate channeling can improve biosynthetic efficiency and has been implicated in the reactions of fusicoccadiene synthase. Here, the authors analyze this bifunctional enzyme complex by cryoEM, cross-linking MS and integrative modeling, providing structural insights into how substrate channeling is achieved.
- Jacque L. Faylo
- , Trevor van Eeuwen
- & David W. Christianson
-
Article
| Open Access Caerulomycin and collismycin antibiotics share a trans-acting flavoprotein-dependent assembly line for 2,2’-bipyridine formation
Caerulomycin and collismycin antibiotics share a trans-acting flavoprotein-dependent assembly line for 2,2’-bipyridine formation
Caerulomycins and collismycins are two types of 2,2’-bipyridine natural products that are biosynthesized via a hybrid NRPS-PKS pathway, but the details of their biosynthesis were unknown. Here, the authors elucidate their biosynthetic pathways, validate the generality of 2,2’-bipyridine formation, and clarify the process for 2,2’-bipyridine furcation.
- Bo Pang
- , Rijing Liao
- & Wen Liu
-
Article
| Open Access Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity
Structures of a non-ribosomal peptide synthetase condensation domain suggest the basis of substrate selectivity
Non-ribosomal peptide synthetases (NRPSs) are multi-modular enzymes assembling complex natural products. Here, the structures of a Thermobifida fusca NRPS condensation domain bound to the substrate-bearing peptidyl carrier protein (PCP) domain provide insight into the mechanisms of substrate selectivity and engagement within the catalytic pocket.
- Thierry Izoré
- , Y. T. Candace Ho
- & Max J. Cryle
-
Article
| Open Access A [6+4]-cycloaddition adduct is the biosynthetic intermediate in streptoseomycin biosynthesis
A [6+4]-cycloaddition adduct is the biosynthetic intermediate in streptoseomycin biosynthesis
Streptoseomycin is a potent antibiotic that contains a pentacyclic 5/14/10/6/6 ring system. Here, the authors report the enzymatic and non-enzymatic steps of the downstream modification of streptoseomycin biosynthesis and show a [6 + 4]-cycloaddition adduct as an unexpected biosynthetic intermediate.
- Kai Biao Wang
- , Wen Wang
- & Hui Ming Ge
-
Article
| Open Access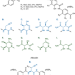 In vivo and in vitro reconstitution of unique key steps in cystobactamid antibiotic biosynthesis
In vivo and in vitro reconstitution of unique key steps in cystobactamid antibiotic biosynthesis
The biosynthetic pathway leading to the production of cystobactamids, a group of myxobacteria-derived topoisomerase inhibitors with potent anti-Gram-negative activity, remains unclear. Here, the authors report in vivo and in vitro evidence for unique steps in cystobactamid biosynthesis.
- Sebastian Groß
- , Bastien Schnell
- & Rolf Müller
-
Article
| Open Access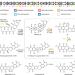 Enzymatic spiroketal formation via oxidative rearrangement of pentangular polyketides
Enzymatic spiroketal formation via oxidative rearrangement of pentangular polyketides
Rubromycin family of natural products belongs to aromatic polyketides with diverse bioactivities, but details of their biosynthesis are limited. Here, the authors report the complete in vitro reconstitution of enzymatic formation of the spiroketal moiety of rubromycin polyketides, driven by flavin-dependent enzymes, and characterize reaction intermediates.
- Britta Frensch
- , Thorsten Lechtenberg
- & Robin Teufel
-
Article
| Open Access Evolution of combinatorial diversity in trans-acyltransferase polyketide synthase assembly lines across bacteria
Evolution of combinatorial diversity in trans-acyltransferase polyketide synthase assembly lines across bacteria
Trans-acyltransferase polyketide synthases are multimodular enzymes that synthesise diverse polyketides. Here, the authors present an algorithm for the global study of their diversity, showing exchange of conserved consecutive modules as a driver of diversification, and guiding the discovery of polyketides.
- Eric J. N. Helfrich
- , Reiko Ueoka
- & Marnix H. Medema
-
Article
| Open Access Gut bacteria are essential for normal cuticle development in herbivorous turtle ants
Gut bacteria are essential for normal cuticle development in herbivorous turtle ants
Microbial symbionts can help their hosts metabolise diverse diets. A study on herbivorous turtle ants identifies the cuticular components which are nitrogen-enriched by gut bacteria, highlighting the role of symbionts in insect evolution.
- Christophe Duplais
- , Vincent Sarou-Kanian
- & Corrie S. Moreau
-
Article
| Open Access Posttranslational chemical installation of azoles into translated peptides
Posttranslational chemical installation of azoles into translated peptides
Azoles are five-membered heterocycles found in peptidic natural products and synthetic peptiodomimetics. Here the authors demonstrate a posttranslational chemical modification method for in vitro ribosomal synthesis of peptides with exotic azole groups at specific positions.
- Haruka Tsutsumi
- , Tomohiro Kuroda
- & Hiroaki Suga
-
Article
| Open Access Engineering and elucidation of the lipoinitiation process in nonribosomal peptide biosynthesis
Engineering and elucidation of the lipoinitiation process in nonribosomal peptide biosynthesis
Nonribosomal lipopeptides contain an acyl chain important for bioactivity, but its incorporation into the peptidyl backbone, mediated by the starter condensation (Cs) domain of nonribosomal peptide synthases, is not fully understood. Here, the authors show that acyl chains of different lengths can be obtained by engineering Cs domains and identify residues that determine the selectivity for acyl chains.
- Lin Zhong
- , Xiaotong Diao
- & Xiaoying Bian
-
Article
| Open Access Thioesterase-mediated side chain transesterification generates potent Gq signaling inhibitor FR900359
Thioesterase-mediated side chain transesterification generates potent Gq signaling inhibitor FR900359
FR900359 (FR) is a Gq protein inhibitor depsipeptide isolated from an uncultivable plant endosymbiont and synthesized by non-ribosomal peptide synthetases. Here, the authors discover a cultivable bacterial FR producer and show that FrsA thioesterase domain catalyses intermolecular transesterification of the FR side chain to the depsipeptide core during biosynthesis, improving Gq inhibition properties.
- Cornelia Hermes
- , René Richarz
- & Max Crüsemann
-
Article
| Open Access Microbial production of multiple short-chain primary amines via retrobiosynthesis
Microbial production of multiple short-chain primary amines via retrobiosynthesis
Short-chain primary amines (SCPAs) are industrially important compounds that are commonly produced under harsh synthetic conditions. Here, the authors report a combination of retrobiosynthesis and precursor selection step for design of biosynthetic pathways leading to production of SCPAs, using valine decarboxylase-expressing Escherichia coli strains.
- Dong In Kim
- , Tong Un Chae
- & Sang Yup Lee
-
Article
| Open Access A partially self-regenerating synthetic cell
A partially self-regenerating synthetic cell
A fundamental function of living systems is regenerating essential components. Here the authors design an artificial cell using a minimal transcription-translation system in microfluidic reactors for sustained regeneration of multiple essential proteins.
- Barbora Lavickova
- , Nadanai Laohakunakorn
- & Sebastian J. Maerkl
-
Article
| Open Access Heme biosynthesis depends on previously unrecognized acquisition of iron-sulfur cofactors in human amino-levulinic acid dehydratase
Heme biosynthesis depends on previously unrecognized acquisition of iron-sulfur cofactors in human amino-levulinic acid dehydratase
Heme biosynthesis depends on iron-sulfur (Fe-S) cluster biogenesis but the molecular connection between these pathways is not fully understood. Here, the authors show that the heme biosynthesis enzyme ALAD contains an Fe-S cluster, disruption of which reduces ALAD activity and heme production in human cells.
- Gang Liu
- , Debangsu Sil
- & Tracey Ann Rouault
-
Article
| Open Access Molecular basis of regio- and stereo-specificity in biosynthesis of bacterial heterodimeric diketopiperazines
Molecular basis of regio- and stereo-specificity in biosynthesis of bacterial heterodimeric diketopiperazines
Bacterial heterodimeric tryptophan-containing diketopiperazines (HTDKPs) are bioactive natural products that are difficult to access chemically. Here, the authors identify a family of three related HTDKP-forming cytochrome P450s and engineer key amino acid residues to produce distinct diketopiperazines frameworks.
- Chenghai Sun
- , Zhenyao Luo
- & Xudong Qu
-
Article
| Open Access Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences
Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences
Large-scale sequencing efforts have uncovered a large number of secondary metabolic pathways, but the chemicals they synthesise remain unknown. Here the authors present PRISM 4, which predicts the chemical structures encoded by microbial genome sequences, including all classes of bacterial antibiotics in clinical use.
- Michael A. Skinnider
- , Chad W. Johnston
- & Nathan A. Magarvey
-
Article
| Open Access α-proteobacteria synthesize biotin precursor pimeloyl-ACP using BioZ 3-ketoacyl-ACP synthase and lysine catabolism
α-proteobacteria synthesize biotin precursor pimeloyl-ACP using BioZ 3-ketoacyl-ACP synthase and lysine catabolism
Biosynthesis of biotin precursor, pimelate moiety, is elucidated in Escherichia coli and Bacillus subtilis, but not understood in alphaproteobacteria. Here, the authors show that BioZ, a 3-ketoacyl-ACP synthase, catalayses pimeloyl-thioester synthesis in alphaproteobacteria using malonyl-ACP and glutaryl-CoA that is derived from lysine degradation.
- Yuanyuan Hu
- & John E. Cronan
-
Article
| Open Access Reprogramming bacterial protein organelles as a nanoreactor for hydrogen production
Reprogramming bacterial protein organelles as a nanoreactor for hydrogen production
The extreme oxygen sensitive character of hydrogenases is a longstanding issue for hydrogen production in bacteria. Here, the authors build carboxysome shells in E. coli and incorporate catalytically active hydrogenases and functional partners within the empty shell for the production of hydrogen.
- Tianpei Li
- , Qiuyao Jiang
- & Lu-Ning Liu
-
Article
| Open Access One-pot biocatalytic route from cycloalkanes to α,ω‐dicarboxylic acids by designed Escherichia coli consortia
One-pot biocatalytic route from cycloalkanes to α,ω‐dicarboxylic acids by designed Escherichia coli consortia
Aliphatic α,ω-dicarboxylic acids (DCAs) are widely used chemicals that are synthesised by multistage chemical oxidations. Here, the authors report an artificially designed biocatalytic cascade for the oxidation of cycloalkanes or cycloalkanols to DCAs in the form of microbial consortia, composed of three Escherichia coli cell modules.
- Fei Wang
- , Jing Zhao
- & Aitao Li
-
Article
| Open Access Oxepinamide F biosynthesis involves enzymatic d-aminoacyl epimerization, 3H-oxepin formation, and hydroxylation induced double bond migration
Oxepinamide F biosynthesis involves enzymatic d-aminoacyl epimerization, 3H-oxepin formation, and hydroxylation induced double bond migration
Oxepinamides are a class of fungal oxepins with biological activities. Here, the authors elucidate the biosynthetic pathway of oxepinamide F from Aspergillus ustus and show that it involves enyme-catalysed oxepin ring formation, hydroxylation-induced double bond migration, epimerization and methylation.
- Liujuan Zheng
- , Haowen Wang
- & Shu-Ming Li
-
Article
| Open Access Uncovering the cytochrome P450-catalyzed methylenedioxy bridge formation in streptovaricins biosynthesis
Uncovering the cytochrome P450-catalyzed methylenedioxy bridge formation in streptovaricins biosynthesis
Streptovaricin C is an antibiotic containing a methylenedioxy bridge (MDB) moiety essential for its activity. Here, the authors show that a P450 monooxygenase StvP2 catalyses MDB formation, report its crystal structure in complex with the substrate, and elucidate mechanistic details of MDB formation.
- Guo Sun
- , Chaoqun Hu
- & Yuhui Sun
-
Article
| Open Access Genetically controlled membrane synthesis in liposomes
Genetically controlled membrane synthesis in liposomes
Controlled membrane synthesis in liposomes is a prerequisite for synthetic systems emulating the fundamental properties of living cells. Here authors present that a de novo synthesized metabolic pathway converts precursors into a variety of lipids, including the constituents of the parental liposome.
- Duco Blanken
- , David Foschepoth
- & Christophe Danelon
-
Article
| Open Access In vitro Cas9-assisted editing of modular polyketide synthase genes to produce desired natural product derivatives
In vitro Cas9-assisted editing of modular polyketide synthase genes to produce desired natural product derivatives
Several different genetic strategies have been reported for the modification of polyketide synthases but the highly repetitive modular structure makes this difficult. Here the authors report on an adapted Cas9 reaction and Gibson assembly to edit a target region of the polyketide synthases gene in vitro.
- Kei Kudo
- , Takuya Hashimoto
- & Kazuo Shin-ya
-
Article
| Open Access Animal biosynthesis of complex polyketides in a photosynthetic partnership
Animal biosynthesis of complex polyketides in a photosynthetic partnership
Complex polyketides are usually produced by microbes, whereas the origin of polyketides found in animals remained unknown. This study shows that sacoglossan animals, such as sea slugs, employ fatty acid synthase-like proteins to produce microbe-like polyketides.
- Joshua P. Torres
- , Zhenjian Lin
- & Eric W. Schmidt
-
Article
| Open Access Human aminolevulinate synthase structure reveals a eukaryotic-specific autoinhibitory loop regulating substrate binding and product release
Human aminolevulinate synthase structure reveals a eukaryotic-specific autoinhibitory loop regulating substrate binding and product release
Mutation of the C-terminal extension of 5′-aminolevulinate synthase 2 (ALAS2) is the molecular cause for X-linked protoporphyria, but the underlying mechanism is unclear. Based on crystal structures and MD simulations of ALAS2, the authors here show how the C-terminal extension regulates ALAS2 activity.
- Henry J. Bailey
- , Gustavo A. Bezerra
- & Wyatt W. Yue
-
Article
| Open Access Minimal lactazole scaffold for in vitro thiopeptide bioengineering
Minimal lactazole scaffold for in vitro thiopeptide bioengineering
Lactazole A is a thiopeptide from Streptomyces lactacystinaeus, encoded by a compact 9.8 kb biosynthetic gene cluster. Here, the authors show a platform for in vitro biosynthesis of lactazole A via a combination of a flexible in vitro translation system with recombinantly produced lactazole biosynthetic enzymes.
- Alexander A. Vinogradov
- , Morito Shimomura
- & Hiroyasu Onaka
-
Article
| Open Access Identity and function of an essential nitrogen ligand of the nitrogenase cofactor biosynthesis protein NifB
Identity and function of an essential nitrogen ligand of the nitrogenase cofactor biosynthesis protein NifB
NifB is a radical SAM enzyme involved in the biosynthesis of the Mo-nitrogenase cofactor, which is responsible for the ambient conversion of N2 to NH3. Here, the authors identify and uncover the function of a His43 residue as an essential nitrogen ligand of NifB in cofactor biosynthesis.
- Lee A. Rettberg
- , Jarett Wilcoxen
- & Markus W. Ribbe
-
Article
| Open Access Gating mechanism of elongating β-ketoacyl-ACP synthases
Gating mechanism of elongating β-ketoacyl-ACP synthases
The formation of C-C bonds in fatty acid and polyketide biosynthesis depends on β-ketoacyl-acyl carrier protein (ACP) synthases (KSs). Here, the authors present structures of E.coli KSs bound to substrate mimetic bearing ACPs, providing insights into the catalytic mechanism underlying C-C bond forming reactions.
- Jeffrey T. Mindrebo
- , Ashay Patel
- & Michael D. Burkart

 Complete biosynthetic pathway to the antidiabetic drug acarbose
Complete biosynthetic pathway to the antidiabetic drug acarbose