Abstract
(-)-epigallocatechin-3-gallate (EGCG) is a well-known cancer chemopreventive agent. The potential mechanisms include regulation of multiple molecules. Carcinogenesis in lung cancer is related to the imbalance of tumor suppressor and oncogene. JWA is a structurally novel microtubule-binding protein and is a potential tumor suppressor. DNA topoisomerase IIα is a nuclear enzyme that governs DNA topology and is usually highly expressed in many types of cancer. It serves as a target of anticancer drugs. In the current study, the regulation of JWA and topoisomerase IIα by EGCG and thereafter the mutual interaction between them was investigated. The results revealed that EGCG up-regulated JWA while decreased topoisomerase IIα expression in both human non-small cell lung cancer (NSCLC) cells and an NSCLC xenograft mice model. There was a negative correlation between JWA and topoisomerase IIα in NSCLC as well as in human NSCLC tissue specimens. Topoisomerase IIα overexpression reduced JWA at the translational level. Meanwhile, JWA-induced topoisomerase IIα degradation was regulated both in the transcriptional and post-translational level. Interestingly, JWA and topoisomerase IIα regulated each other in the cells arrested in G2/M. Furthermore, JWA and topoisomerase IIα synergistically affected NCI-H460 cells invasion. These results may serve a novel mechanism for cancer prevention.
Similar content being viewed by others
Introduction
Lung cancer is a leading cause of cancer death. Non-small-cell lung cancer (NSCLC) represents approximately 85% of lung cancer cases, with a world-wide annual incidence of approximately 1.3 million1. Advances in the understanding of specific molecular abnormalities can provide new strategies for personalized lung cancer treatment including gene amplifications (e.g., MET, FGFR1), mutations (e.g., EGFR, p53) and fusions (e.g., EML4-ALK)2. Chemoprevention is a promising strategies interfering carcinogenesis. EGCG, a major active polyphenol, has captured much attention as a potential cancer chemopreventive agent3,4. Previous studies have revealed the possible molecular mechanisms of EGCG to control lung cancer insurgence5,6,7.
JWA is known as adenosine diphosphate-ribosylation-like factor 6 interacting protein 5 (ARL6ip5) in GenBank (AF070523). It was initially cloned from human tracheal bronchial epithelial cells after treatment with all-trans retinoic acid (ATRA). JWA encodes a structurally novel microtubule-associated protein, which regulates cancer cells differentiation and apoptosis induced by multiple chemicals8,9. JWA responses to environmental stimulations including heat shock and H2O2-induced oxidative stress10,11. It has been reported that JWA may serve as a repair protein by regulating base excision repair (BER) protein XRCC112. On the other hand, JWA is known as a novel tumor suppressor which regulates tumor angiogenesis by suppressing matrix metalloprotein (MMP) and inhibiting cell invasion via focal adhesion kinase (FAK/PTK2)13. Further investigations indicate that JWA can work as a cooperator with p53, MDM2 or XRCC1 to improve predictive potency in gastric cancer14,15,16. Moreover, JWA sensitizes p-glycoprotein-mediated drug resistance to anticancer drug etoposide (topoisomerase II inhibitor)17.
DNA topoisomerases are ubiquitous nuclear enzymes that govern DNA topology and fundamental DNA processes involved in DNA replication, transcription, chromosome condensation and recombination18. There are two main types of the enzyme, catalyzing transient breaks in one (type I) or both (type II) strands of DNA. In the topoisomerase II family, topoisomerase IIα and topoisomerase IIβ are homologous sharing extensive amino acid sequence identity (~70%). However, the two isoforms have distinct patterns of expression19. Topoisomerase IIα is cell cycle-dependent and primarily expresses in rapidly proliferating cells. High levels of this isoform are found in many types of cancer, therefore it is a cancer target in clinical application20,21. The chemotherapeutic properties are attributed primarily to topoisomerase IIα22. Although topoisomerase II-mediated DNA cleavage has been recognized as an effective molecular target for many antitumor drugs23, frequently experienced occurrence of serious side effects of these molecules during therapy have been reported24.
It is reported that EGCG is redox-dependent topoisomerase II poison25,26. It enhances DNA cleavage and affects topoisomerase activity mediated by both enzyme isoforms27,28. In the present study, we find EGCG could also suppress topoisomerase IIα expression. Interestingly, it also up-regulated JWA. The underlying mechanism of the relationship between JWA and topoisomerase IIα was investigated. Whether EGCG participated in the regulation of JWA and topoisomerase IIα in NSCLC cells was explored. Furthermore, the combination of JWA and topoisomerase IIα might serve as a novel candidate prognostic biomarker for NSCLC.
Results
EGCG induced expression of JWA in NSCLC cells
Firstly, the effect of EGCG on JWA expression was investigated in NSCLC cell lines. Total RNA or protein from A549 and NCI-H460 cells treated with indicated concentration of EGCG was isolated respectively. Western blot analysis was used to detect endogenous and exogenous JWA protein level. As shown in Fig. 1a, EGCG up-regulated endogenous JWA protein level in NCI-H460 cells in a dose-dependent manner. When the same concentrations of EGCG were treated to the cells transfected with Flag-JWA plasmid, the exogenous JWA protein level, as tested by anti-Flag antibody, also increased. Then, real-time PCR was preformed to examine JWA mRNA expression. As shown in Fig. 1b, EGCG increased JWA messenger RNA (mRNA) level as well in NCI-H460 cells. Meanwhile, the same effect of EGCG on JWA expression was observed in A549 cells (Fig. 1c,d).
EGCG regulated the expression of JWA and topoisomerase IIα (topo IIα) in vitro (NCI-H460 and A549 cells) and in vivo.
(a) Cells transfected with Flag-JWA (4 μg) or without transfection were incubated with EGCG (20–40 μM) for 24 h. JWA protein expression was assessed by Western blot analysis. β-actin expression served as a loading control. (b) NCI-H460 cells were treated with EGCG (20–40 μM) for 24 h and total cellular RNA was extracted. mRNA level of JWA was detected by real-time PCR. GAPDH was used as an internal control. (c) Western blot analysis of the protein level of JWA in EGCG-treated A549 cells for 24 h. β-actin expression served as a loading control. (d) After A549 cells were incubated with EGCG (20–40 μM) for 24 h, total RNAs were prepared and real-time PCR was applied to measure the JWA mRNA level. GAPDH was used as an internal control. (e) NCI-H460 cells were transfected with or without Flag-topoisomerase IIα plasmid (4 μg) and then treated with EGCG (20–40 μM) for 24 h. Protein from cell was subjected to western blot analysis. β-actin expression was served as a loading control. (f) Total RNAs from NCI-H460 cells incubated with EGCG (20–40 μM) for 24 h were extracted and subjected to real-time PCR using primer for topoisomerase IIα. GAPDH was used as an internal control. (g) A549 cells were treated with EGCG (20–40 μM) for 24 h and then protein from cell lysate was subjected to western blot analysis. β-actin expression was served as a loading control. (h) A549 cells were incubated in the absence or presence of EGCG (20–40 μM) for 24 h. Then cells were lysed for the detection expression of topoisomerase IIα mRNA by real-time PCR. GAPDH was used as an internal control. The A549 xenograft nude mice model was established and treated with EGCG or normal saline. (i) Tumor size was checked twice per week. (j) Protein obtained from tumor tissues was subjected to western blot. β-tubulin expression was served as a loading control. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01.
EGCG suppressed topoisomerase IIα expression in NSCLC cells.
As EGCG is an inhibitor of topoisomerase II in leukaemia and hamster ovary cells26,29, whether EGCG shows inhibitory effect on topoisomerase II expression was investigated in NCI-H460 and A549 cells. Cells were treated with EGCG at the indicated concentrations for 24 h and followed by western blot assay. As shown in Fig. 1e,g, EGCG significantly inhibited protein expression of topoisomerase IIα in NCI-H460 and A549 cells. We further determined the effect of EGCG on expression of topoisomerase IIα mRNA by real-time PCR, The results revealed that topoisomerase IIα mRNA expression was closed to the level of control in both of the cells (Fig. 1f,h). The protein level of topoisomerase IIα was down-regulated by EGCG while mRNA level was not changed, which indicating post-transcription regulation of topoisomerase IIα by EGCG.
EGCG increased the expression of JWA while down-regulated topoisomerase IIα levels in tumor-bearing nude mice
An A549 xenograft nude mice model was established to confirm the influence of EGCG on JWA and topoisomerase IIα expression in vivo. As shown in Fig. 1i, the tumor growth was repressed significantly by EGCG treatment for 30 days, compared to the control group. Treatment of EGCG altered the expression of JWA and topoisomerase IIα. Topoisomerase IIα protein expression was reduced in EGCG-treated mice compared with the control. On the contrary, JWA levels were increased after EGCG treatment (Fig. 1j). These results were consistent with the above experiments in vitro. These findings indicated that there was a negative correlation between JWA and topoisomerase IIα protein expression upon EGCG treatment.
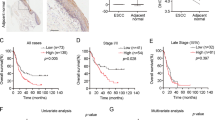
The expression of JWA and topoisomerase IIα in lung cancer versus adjacent normal tissues
Four pairs of human NSCLC samples, including primary lung cancer tissues and matched non-cancerous tissues were selected to test JWA and topoisomerase IIα protein expression by western blot. Elevated expression of topoisomerase IIα and decreased JWA level were observed in lung cancer tissues compared with the paired normal tissues (Fig. 2a,b). The results showed a negative correlation between JWA and topoisomerase IIα expression in lung cancer versus non-cancer tissues.
There was a negative correlation between JWA and topoisomerase IIα protein expression.
(a) and (b) Expression of topoisomerase IIα was elevated and that of topoisomerase IIα was reduced in lung cancer tissues compared with those in matched normal tissues. β-Tubulin expression was served as a loading control. JWA and topoisomerase IIα expressions in cancer tissues (T) and paired non-cancerous normal tissues (N) of lung cancer patients were analyzed by Western blotting. The level of each protein was normalized against β-tubulin. NCI-H460 cells were transiently transfected with Flag-JWA or Flag- topoisomerase IIα plasmid (1–4 μg). Whole-cell extracts were prepared 24 h after transfection and the expression of target proteins and mRNAs was examined. As analyzed by western blot assay, topoisomerase IIα levels were decreased after transfection with JWA plasmid (c) Also, overexpression of topoisomerase IIα does dependently inhibited JWA protein expression (d) β-actin was used for the protein loading control. Total RNAs were isolated from NCI-H460 cells transfected with Flag-JWA or Flag- topoisomerase IIα plasmid (4 μg) and subjected to real-time PCR. JWA transfection suppressed topoisomerase IIα mRNA expression (e) The level of JWA mRNA was down-regulated in topoisomerase IIα overexpressed NCI-H460 cells (f) GAPDH was used as an internal control. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01.
JWA and topoisomerase IIα reciprocally down-regulated in NSCLC cells
Since EGCG exhibited opposite effect on expression of JWA and topoisomerase IIα, we therefore postulated if there existed a converse-regulation between JWA and topoisomerase IIα. To verify this hypothesis, NCI-H460 cells were transfected with plasmids to overexpress JWA or topoisomerase IIα. The western blot analysis showed that JWA overexpression suppressed topoisomerase IIα levels in a dose-dependent manner (Fig. 2c). Interestingly, topoisomerase IIα dose-dependently inhibited JWA protein expression (Fig. 2d).
We further examined the topoisomerase IIα mRNA expression after transfection of JWA. Total RNAs were isolated from the cells and subjected to real-time PCR. As shown in Fig. 2e, transfection of JWA suppressed topoisomerase IIα mRNA expression. Also, the levels of JWA mRNA were decreased in NCI-H460 cells which topoisomerase IIα was overexpressed (Fig. 2f).
Since the above results showed that JWA and topoisomerase IIα down-regulated each other at both mRNA and protein levels, we further confirmed whether the proteasome or lysosome pathway was responsible for the negative regulation between JWA and topoisomerase IIα. When NCI-H460 cells were treated with the proteasome inhibitor MG132, we observed that down-regulation of topoisomerase IIα in JWA overexpressed cells was blocked (Fig. 3a). Leupeptin, a lysosome inhibitor, was able to reverse the decreased topoisomerase IIα triggered by JWA (Fig. 3b). However, when the cells were transfected with topoisomerase IIα in the presence of leupeptin or MG-132, JWA level still decreased (Fig. 3c,d). These data revealed that both lysomal and proteasomal pathway were involved in JWA-mediated suppression of topoisomerase IIα. Nevertheless, topoisomerase IIα-induced JWA degradation might not occurred in the proteasome or lysosome.
Different degradation pathway regulated the inverse interaction between topoisomerase IIα and JWA protein expression.
(a) and (b) NCI-H460 cells were transiently transfected with Flag-JWA plasmid (2.5 μg) for 24 h, incubated with (+) or without (-) MG-132 (5 μM) for 8 h or leupeptin (5 μM) for 20 h. Western blotting was carried out to confirm the level of topoisomerase IIα. (c) and (d) NCI-H460 cells were transiently transfected with Flag- topoisomerase IIα plasmid (2.5 μg) for 24 h and co-treated with (+) or without (-) MG-132 (5 μM) for 8 h or leupeptin (5 μM) for 20 h. JWA protein was detected by anti-JWA antibody. β-actin expression served as a loading control. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01.
The amino acid sequence of JWA ranging from 90 to 188 played a crucial role in topoisomerase IIα degradation
To elucidate which fragments of JWA acted in topoisomerase IIα degradation, four fragments were constructed based on JWA functional domain, of which amino acid sequences were ranged from 1 to 60 (JWA-1), 1 to 90 (JWA-2), 1 to 140 (JWA-3) and 141–188 (JWA-4) (Fig. 4a). After transient transfection of these constructs’ plasmids into NCI-H460 cells, we examined the topoisomerase IIα protein expression by western blot analysis. It revealed significant down-regulation of topoisomerase IIα with JWA-3 (1–140) and JWA-4 (141–188) fragments compared with the control (Fig. 4b). However, no makeable difference was observed in cells transfected with JWA-1 (1–60) and JWA-2 (1–90). These results indicated that the region of JWA amino acid sequence between 90 and 188 played an important function for topoisomerase IIα degradation.
The deletion of 90–188 amino acid of JWA played a major function for topoisomerase IIα degradation.
(a) Schematic showed the domains of full length and indicated fragments JWA protein. “Dark grey” represented the TMD of JWA. Amino acid sequences ranged from 1 to 60 for JWA-1, 1 to 90 for JWA-2, 1 to 140 for JWA-3 and 141–188 for JWA-4. (b) NCI-H460 cells were transiently transfected with full-length Flag-JWA plasmid and four different fragments of JWA plasmid respectively. Whole-cell extracts were prepared 24 h after transfection and topoisomerase IIα expression was measured by western blotting. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01.
The mutual regulation of JWA and topoisomerase IIα was occurred in G2/M
Considering both JWA and topoisomerase IIα are related to tumor cell proliferation and division, we speculated that their mutual interaction might be regulated by cell cycle progression. By using serum starving to arrest cells to G0 phase (Fig. 5a), the expression of JWA was down-regulated after 24 h and then reversed 6 h later. The change of topoisomerase IIα protein was as the same as JWA (Fig. 5c and d). Nocodazole, a mitotic inhibitor, has the effect on majority of NCI-H460 cell synchronization in G2/M phase (Fig. 5b)30. In our study, the protein levels of JWA were decreased while increased of topoisomerase IIα when treated with 200 nM of nocodazole for indicated time (Fig. 5e,f).
Cell cycle modulated the inverse regulation of topoisomerase IIα and JWA protein expression.
NCI-H460 cells were plated on 60 mm plates in DMEM (10% fetal bovine serum), which was changed to starving DMEM (without serum) for 24 h or 30 h. (a) Flow cytometric analysed the change of cell cycle. And the protein levels of JWA and topoisomerase IIα were examined by Western blotting. (c) and (d) Then NCI-H460 cells were treated with 200 nM nocodazole, respectively for 12 h, 18 h and 24 h. (b) Flow cytometric analysed the cell cycle. (e) and (f)The expression of JWA and topoisomerase IIα were examined by Western blotting. β-actin expression served as a loading control. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01 and ***p < 0.001.
Synergistic effect of JWA and topoisomerase IIα on suppression of lung cancer cell migration and invasion
As described above, topoisomerase IIα is overexpressed in many types of cancer, its down-regulation is a therapeutic mechanism of cancer. Conversely, JWA is usually served as a tumor suppressor and its level is relatively lower in many types of tumor. To study the potential synergistic role of JWA and topoisomerase IIα in lung carcinogenesis, we first investigated the involvement of topoisomerase IIα and JWA in NCI-H460 cells migration using shRNA-JWA, siRNA-topoisomerase IIα, JWA, topoisomerase IIα plasmids and the control vector. A scratch migration assay revealed that topoisomerase IIα deficiency and JWA overexpression cells showed a marked delay in wound closure; Moreover, the capacity of cell migration was increased in sh-JWA transfected cells. Co-transfection of topoisomerase IIα and sh-JWA further accelerated the wound closure (Fig. 6a).
EGCG regulated the interaction between JWA and topoisomerase IIα and their synergistic effect on inhibition of NCI-H460 cells migration and invasion.
(a) NCI-H460 cells were transfected with siRNA-topoisomerase IIα (100 pmol), shRNA-JWA, JWA and topoisomerase IIα plasmids (4 μg) as well as the control vector. Migration ability of the cells at various time points after transfection (24 h, 48 h) was assessed by scratch migration assay. (b) The JWA or topoisomerase IIα plasmid (4 μg) was transiently transfected into NCI-H460 cells. 24 hours later, target proteins in cell lysates were detected by immunoblotting using antibodies against MMP-2/9, N- cadherin, ZEB1, slug, snail and E-cadherin. β-actin expression served as a loading control. (c) and (d) NCI-H460 cells were transfected with Flag-JWA , Flag- topoisomerase IIα and Flag-vector plasmids, (4 μg) in the presence or absence of EGCG (40 μM) for 24 h. The cells were harvested and lysed for the detection expression of JWA or topoisomerase by Western blot analysis. β-actin expression served as a loading control. Error bars represent the mean ± SD of triplicate experiments. Statistical differences to the controls were shown as *p < 0.05, **p < 0.01.
EMT (Epithelial-mesenchymal transition) endows cells with migration and invasion abilities31. Considering that downregulation of JWA affected NCI-H460 cells migration, we sought to determine whether JWA affected the expression of EMT signatures. We observed that the expression of MMP-2/9, N-cadherin, ZEB1, slug and snail was down-regulated while E-cadherin was up-regulated after transfection of JWA to NCI-H460 cells. As expected, when cells overexpressed topoisomerase IIα, the levels of MMP-2/9, N- cadherin, ZEB1, slug and snail was higher than that of control, but E-cadherin was at the lower level (Fig. 6b). Taken together, JWA and topoisomerase IIα had a synergistic effect on inhibition of lung cancer cell migration and invasion.
EGCG regulated the interaction between JWA and topoisomerase IIα
To confirm whether EGCG participated in regulating the cross-talk between JWA and topoisomerase IIα in NCI-H460 cells, cells were transfected with JWA or topoisomerase IIα plasmids, in the presence or absence of EGCG treatment. When cells were transfected with topoisomerase IIα in the presence of EGCG, the down-regulated JWA level was reversed (Fig. 6c). Meanwhile, EGCG was able to further decrease topoisomerase IIα protein expression induced by JWA (Fig. 6d). These results indicated that EGCG was involved in regulating the interplay between JWA and topoisomerase IIα in NCI-H460 cells.
Discussion
As a powerful cancer chemopreventive agent, EGCG can regulate series of molecular. Topoisomerase IIα is one of those targets. Previous studies showed that EGCG is a topoisomerase inhibitor and poison to topoisomerase II25,26,27. In the present study, we found that EGCG could suppress topoisomerase IIα expression in the post-transcriptional level in NSCLC in vitro and in vivo. We also found that EGCG could induce JWA expression. JWA is a potential tumor suppressor. The inverse regulation of topoisomerase IIα and JWA by EGCG may add a novel chemopreventive property to EGCG. It is also interesting to find that JWA and topoisomerase IIα are reciprocally down-regulated in several tumour cell lines including NSCLC cells. To our knowledge, this is the first report uncovering the converse-correlation between JWA and topoisomerase IIα and the potential mechanisms involved.
There are multiple cis-action elements, such as stress response element (SRE) and heat shock response element (hRE) existed within the identified promoter sequence of JWA8,10. JWA expression is dependent upon generation of intracellular ROS and protects cells against ROS-associated DNA damage10,11,12. EGCG can be a powerful antioxidant to affect intracellular ROS level. The induction of JWA by EGCG could be due to its antioxidant property. Previous research found that EGCG could affect topoisomerase enzyme in a redox-dependent way26. Our unpublished data also showed that antioxidants NAC and H2O2 affected JWA and topoisomerase IIα expression. These data indicated that redox ingredients, such as ROS, might regulate the expression of JWA and topoisomerase IIα.
We observed that JWA and topoisomerase IIα were mutually down-regulated in various cancer cell lines including NSCLC cells and human NSCLC specimens (Fig. 2 and Supplement Figure S1). Overexpression of topoisomerase IIα did not affect the stability of JWA in the cells incubated with MG-132 or leupeptin. Topoisomerase IIα regulated JWA expression at the transcriptional level. Conversely, JWA-mediated topoisomerase IIα degradation was also modulated in a post-translation level and via proteasome and lysosome pathways. The activity of topoisomerase IIα is regulated by posttranslational modification or by interaction with regulatory proteins32. Topoisomerase contains a less conserved and largely disordered C-terminal domain (CTD). It is endowed with a main function associated with posttranslational modification and by interaction with regulatory proteins which may result in changes in the half-life of the protein33. Considering these data, it is worthy studying whether JWA influenced the CTD of topoisomerase IIα in the future. Besides, JWA is translocated into the nucleus by the carrier protein XRCC1 and protects XRCC1 protein from ubiquitination by proteasome12. Nevertheless, little research has been conducted concerning the degradation mechanisms of JWA.
The full length cDNA sequence of JWA contains 2114 nucleosides and a 567 base pairs of open reading frame which encoding 188 amino acids. In JWA protein structure, there are three transmembrane domains (TMD) which include 23 amino acids in each domain (36–58, 64–86, 108–130). Two protein kinase C (PKC) phosphorylational sites (SDR-SLR) are found at the both side of TMD. Beside the first TMD, the other two TMDs possess secondary structures such as random coils and extend strands. Based on JWA structural domain, we built up JWA protein with four fragments (1–60, 1–90, 1–140, 141–188) for identification of binding sites between JWA and topoisomerase IIα. We found that the 90–188 amino acid of JWA played a major function for topoisomerase IIα degradation. It was noteworthy that the third TMD (108–130) was included in 90–188 amino acids. The effect of the TMD of JWA on topoisomerase IIα degradation deserves to be investigated. Future studies will also be needed to determine which, if any, of sites are necessary for the interaction between JWA and topoisomerase IIα.
The conversed correlation of JWA and topoisomerase IIα led us to explore the direct interaction between them. Usually, topoisomerase IIα is located in nucleus while JWA largely presented in cytoplasm12,34. As Supplement Fig. 2 showed, there was no overlap of JWA localization with topoisomerase IIα. It also failed to detect complex formation between JWA and topoisomerase by immunoprecipitation (Supplement Figure S3). These results indicated that there was no direct interaction between JWA and topoisomerase IIα. It led us speculate whether cell cycle affect their regulation. Topoisomerase IIα is a critical regulator of G2/M transition and highly expressed at this phase. Defects in topoisomerase IIα can lead to a delay in cell cycle progression19,34. As described above, JWA protein is a novel microtubule-associated protein. The building block of a microtubule is the tubulin subunit that is a heterodimer of α- and β-tubulin. JWA binds to β-tubulin strongly and has completely parallel intracellular distributions35. The preliminary data also show that JWA is associated structurally with cell shape, movement and mitosis. It is reasonable that JWA may exert similar functions as microtubule or as a partner of microtubule. In the current study, when cells were arrested in G2/M, JWA and topoisomerase IIα showed an opposite trend in protein expression. However, they had the synchronous expression pattern in serum-starved cells arrested in G0. This indicated that the converse regulation of JWA and topoisomerase IIα was occurred in G2/M which might be controlled by mitosis process. Detailed evidence needs to be provided by further investigation.
An increasing body of evidence demonstrates that down-regulation of JWA increases malignant phenotypes in a variety of cancers such as sarcomas and cervical carcinoma9,36. Cancer cells with EMT signatures, as evidenced by the decrease of cell adhesion molecules and overexpression of EMT inducers, display increased capability of metastasis37,38. In hepatocellular carcinoma cells, JWA down-regulation enhances cancer progression through the induction of MMP-2 activity and elevated FAK expression13. Also, JWA suppresses tumor angiogenesis via Sp1-activated MMP-2 in human gastric cancer39. The data presented here, indicated that knockdown of topoisomerase IIα and overexpression of JWA suppressed lung cancer cell migration and invasion abilities, which might be partially ascribed to elevated E-cadherin expression and decreased MMP-2/9, N- cadherin, ZEB1, slug and snail levels.
In summary, this work uncovers a possible mechanism for the inverse correlation between JWA and topoisomerase IIα, in which it could be regulated by EGCG in NSCLC. Although a definite co-action of these two proteins is yet to be proven clearly, these findings may provide potential chemoprevention targets for NSCLC. Furthermore, it also leads to a new strategy of EGCG in lung cancer prevention.
Methods
Cell culture and reagents
NSCLC A549 and NCI-H460 cells were purchased from the Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank (Shanghai, China) .Cell lines have been tested and authenticated by the centre. These cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, GIBCO, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY) and 1% penicillin/streptomycin (Beyotime Institute of Biotechnology, Shanghai, China). Cells were maintained at 37 °C in a humidified 5% CO2 atmosphere. EGCG were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nocodazole, MG132 and Leupeptin were obtained from Beyotime Institute of Biotechnology (Shanghai, China).
Plasmids and transient transfection
pIRES-Flag-topoisomerase IIα plasmid was provided by Dr. Lou (Mayo Clinic, MN, USA). The FLAG-JWA 1 (1–60), FLAG –JWA 2 (1–90), FLAG –JWA 3 (1–140), FLAG-JWA 4 (141–188), Flag-JWA, FLAG-vector, shJWA and shJWA vectors were provided by Dr. Jianwei Zhou (Nanjing Medical University, Nanjing, China). Transient transfection was carried out with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, Calif, USA). All steps were performed following the manufacturer’s instructions.
Cells (2 × 105) were transfected with predesigned human topoisomerase IIα siRNA or siRNA control (100 pM) (RiboBio, Guangzhou, China) in 35-mm culture plates using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, Calif, USA).
Tissue specimens
Institutional approval was obtained from the review boards of Nanjing Medical University prior to this study. NSCLC tissues and matched non-cancerous tissues were obtained from postoperative patients in the Nanjing First Hospital. Tissues were grinded in liquid nitrogen and then extracted protein or RNA for analysis. Written informed consent was obtained from each patient prior to tissue acquisition. Institutional approval was obtained from the Ethical Review Board of Nanjing Medical University (Nanjing, China) prior to this study. Experiment was carried out in accordance with the approved guidelines.
Serum starvation assay
NCI-H460 cells were cultured in normal medium (DMEM with 10% fetal bovine serum). When cells were 70% confluent, they were then serum-starved for the indicated time (DMEM without serum). Protein was harvested and used for Western blot analysis.
Western blot analysis
Total cell lysates were prepared with a lysis buffer including 1 mM DTT, 0.1% protease inhibitor and 5 mM PMSF (KeyGEN, Nanjing, China). Protein concentration was checked by BCA Protein Assay Kit (Beyotime, China). The precipitated proteins in the cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, USA). The primary antibodies were: rabbit polyclonal anti-topoisomerase IIα (Abcam, Cambridge, Britain), anti-anti-N-cadherin (Santa Cruz Biotechnology, USA), anti-snail (Affinity Biosciences, USA), anti-slug (Affinity Biosciences, USA), anti-ZEB1 (Affinity Biosciences, USA), anti-E-cadherin (Santa Cruz Biotechnology, USA), anti-MMP2/9 (Abcam, Cambridge, Britain), β-actin (BOSTER, Wuhan, China), monoclonal anti-flag (sigma, St. Louis, USA), anti-anti-JWA was kindly provided by Dr. Jianwei Zhou (Nanjing Medical University, Nanjing, China). Horseradish peroxidase-conjugated affiniPure goat anti-rabbit or anti-mouse IgG (ZSGB-BIO, China) was the second antibody. Immunoreactive proteins were visualized using the Chemiluminesence Western blotting detection reagents (Cell Signaling Technology, Danvers, MA, USA).
Real-Time Polymerase Chain Reaction
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RNA (500 ng) was used for the reverse transcription reaction using the PrimeScriptTM RT Master Mix (TaKaRa Bio Technology). Real-time PCR was performed using SYBRR Premix Ex TaqTMII (TaKaRa BioTechnology) on Applied Biosystems 7300 Real Time PCR System (Applied Biosystems, Foster City, CA) according to manufacturer’s protocol. Expression of mRNA was calculated using the 2−△△Ct method and normalization with GAPDH. Primer sequences are listed below: 5’- CAAGGTCATCCATGACAACTTTG-3’ (forward) and 5’- GTCCACCACCCTGTTGCTGTAG -3’ (reverse) for GAPDH; 5’-TCATGTTGGCGAGCTATTTCC -3’ (forward) and 5’- AGGTTCCGAAGTCTCAACGAT -3’ (reverse) for JWA; 5’- ggcccaagtccagttaaaca -3’ (forward) and 5’-CAGAAAACGATGTCGCAGAA -3’ (reverse) for topoisomerase IIα.
Scratch migration assay
H460 cells were transfected in 6-well plate. After transfection for 48 h (time 0), the cells were scraped with a sterile 10 μl pipette tip across each well and washed with phosphate buffered saline to remove loose cells. Cell migration into the empty space was followed after 24 h and 48 h and photographed. The wound closure was calculated by the average distance between the two wound edges.
Tumor xenograft model
A total of 16 (3–5wk old) female BALB/c nude mice were obtained from Slac Laboratory Animal (Shanghai, China). A549 cells (5 × 106) were harvested and injected into the dorsal of the mice. The tumor size was checked twice per week. Tumor volume was calculated using the following formula: volume (mm3) = length × width × width/2. When the average volume reached 100 mm3, the mice were randomly divided into 2 groups (8 mice/group) as follows: control (normal saline, 0.1 ml/10 g), EGCG (20 mg/kg). Agents were administered twice a week for 4 weeks through intraperitoneal injection. All animals handled in strict accordance with procedures approved by The Center for Hygienic Analysis and Detection of Nanjing Medical University and the Ethical Review Board was acquired prior to this study.
Statistical analysis
Data were presented as the mean ± standard deviation (SD) of three independent experiments. Comparisons between quantitative variables were performed using a student’s two-tailed non-paired t-test or one-way analysis of variance (ANOVA). Statistical significance was set at *P < 0.05, **P < 0.01 and ***P < 0.001. Statistical analysis was carried out using the SPSS 17.0 software.
Additional Information
How to cite this article: Li, Y. et al. EGCG regulates the cross-talk between JWA and topoisomerase IIα in non-small-cell lung cancer (NSCLC) cells. Sci. Rep. 5, 11009; doi: 10.1038/srep11009 (2015).
References
Jemal, A. et al. Global cancer statistics. CA Cancer J Clin. 61, 69–90 (2011).
Pao, W. & Girard, N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 12, 175–180 (2011).
Yang, C. S. et al. Molecular targets for the cancer preventive activity of tea polyphenols. Mol Carcinog. 45, 431–435 (2006).
Yang, C. S. & Wang, H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 55, 819–831 (2011).
Wang, H., Bian, S. & Yang, C. S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis. 32, 1881–1889 (2011).
Yang, C. S., Liao, J., Yang, G. Y. & Lu, G. Inhibition of lung tumorigenesis by tea. Exp Lung Res. 31, 135–144 (2005).
Ko, H. et al. TGF-beta1-induced epithelial-mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in human A549 lung cancer cells. Cancer Lett. 335, 205–213 (2013).
Huang, S. et al. JWA, a novel signaling molecule, involved in all-trans retinoic acid induced differentiation of HL-60 cells. J Biomed Sci. 13, 357–371 (2006).
Chen, H. et al. JWA as a functional molecule to regulate cancer cells migration via MAPK cascades and F-actin cytoskeleton. Cell Signal. 19, 1315–1327 (2007).
Chen, R. et al. Identification of JWA as a novel functional gene responsive to environmental oxidative stress induced by benzo[a]pyrene and hydrogen peroxide. Free Radic Biol Med. 42, 1704–1714 (2007).
Zhou, J., Ye, J., Zhao, X., Li, A. & Zhou, J. JWA is required for arsenic trioxide induced apoptosis in HeLa and MCF-7 cells via reactive oxygen species and mitochondria linked signal pathway. Toxicol Appl Pharmacol. 230, 33–40 (2008).
Wang, S. et al. JWA regulates XRCC1 and functions as a novel base excision repair protein in oxidative-stress-induced DNA single-strand breaks. Nucleic Acids Res. 37, 1936–1950 (2009).
Wu, X. et al. Downregulation of JWA promotes tumor invasion and predicts poor prognosis in human hepatocellular carcinoma. Mol Carcinog. 53, 325–336 (2014).
Liu, X. et al. Synergistic role between p53 and JWA: prognostic and predictive biomarkers in gastric cancer. PLoS One. 7, e52348 (2012).
Ye, Y. et al. MDM2 is a useful prognostic biomarker for resectable gastric cancer. Cancer Sci. 104, 590–598 (2013).
Wang, S. et al. Prognostic and predictive role of JWA and XRCC1 expressions in gastric cancer. Clin Cancer Res. 18, 2987–2996 (2012).
Zhang, Y. et al. JWA sensitizes P-glycoprotein-mediated drug-resistant choriocarcinoma cells to etoposide via JNK and mitochondrial-associated signal pathway. J Toxicol Environ Health A. 72, 774–781 (2009).
Corbett, K. D. & Berger, J. M. Structure, molecular mechanisms and evolutionary relationships in DNA topoisomerases. Annu Rev Biophys Biomol Struct. 33, 95–118 (2004).
Chen, S. H., Chan, N. L. & Hsieh, T. S. New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem. 82, 139–170 (2013).
Wesierska-Gadek, J. & Skladanowski, A. Therapeutic intervention by the simultaneous inhibition of DNA repair and type I or type II DNA topoisomerases: one strategy, many outcomes. Future Med Chem. 4, 51–72 (2012).
Lan, J. et al. TOP2A overexpression as a poor prognostic factor in patients with nasopharyngeal carcinoma. Tumour Biol. 35, 179–187 (2014).
Chen, W., Qiu, J. & Shen, Y. M. Topoisomerase IIalpha, rather than IIbeta, is a promising target in development of anti-cancer drugs. Drug Discov Ther. 6, 230–237 (2012).
Li, Y. et al. Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol Sci. 118, 435–443 (2010).
Mistry, A. R. et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 352, 1529–1538 (2005).
Bandele, O. J. & Osheroff, N. (-)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem Res Toxicol. 21, 936–943 (2008).
Lopez-Lazaro, M., Calderon-Montano, J. M., Burgos-Moron, E. & Austin, C. A. Green tea constituents (-)-epigallocatechin-3-gallate (EGCG) and gallic acid induce topoisomerase I- and topoisomerase II-DNA complexes in cells mediated by pyrogallol-induced hydrogen peroxide. Mutagenesis. 26, 489–498 (2011).
Suzuki, K., Yahara, S., Hashimoto, F. & Uyeda, M. Inhibitory activities of (-)-epigallocatechin-3-O-gallate against topoisomerases I and II. Biol Pharm Bull. 24, 1088–1090 (2001).
Austin, C. A., Patel, S., Ono, K., Nakane, H. & Fisher, L. M. Site-specific DNA cleavage by mammalian DNA topoisomerase II induced by novel flavone and catechin derivatives. Biochem J. 282, 883–889 (1992).
Neukam, K., Pastor, N. & Cortes, F. Tea flavanols inhibit cell growth and DNA topoisomerase II activity and induce endoreduplication in cultured Chinese hamster cells. Mutat Res. 654, 8–12 (2008).
Masuda, A., Maeno, K., Nakagawa, T., Saito, H. & Takahashi, T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol. 163, 1109–1116 (2003).
Mikami, S. et al. Expression of Snail and Slug in renal cell carcinoma: E-cadherin repressor Snail is associated with cancer invasion and prognosis. Lab Invest. 91, 1443–1458 (2011).
Chikamori, K. et al. DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr Cancer Drug Targets. 10, 758–771 (2010).
Vos, S. M., Tretter, E. M., Schmidt, B. H. & Berger, J. M. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol. 12, 827–841 (2011).
Wang, Y., Azuma, Y., Moore, D., Osheroff, N. & Neufeld, K. L. Interaction between tumor suppressor adenomatous polyposis coli and topoisomerase IIalpha: implication for the G2/M transition. Mol Biol Cell. 19, 4076–4085 (2008).
Shen, L., Xu, W., Li, A., Ye, J. & Zhou, J. JWA enhances As(2)O(3)-induced tubulin polymerization and apoptosis via p38 in HeLa and MCF-7 cells. Apoptosis. 16, 1177–1193 (2011).
Cunha, I. W. et al. Identification of genes associated with local aggressiveness and metastatic behavior in soft tissue tumors. Transl Oncol. 3, 23–32 (2010).
Wan, L., Pantel, K., Kang, Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 19, 1450–1464 (2013).
Fang, J. H. et al. A novel vascular pattern promotes metastasis of hepatocellular carcinoma in an EMT-independent manner. Hepatology. (2015). [Epub ahead of print].
Chen, Y. et al. JWA suppresses tumor angiogenesis via Sp1-activated matrix metalloproteinase-2 and its prognostic significance in human gastric cancer. Carcinogenesis. 35, 442–451 (2014).
Acknowledgements
We thank Dr. Jianwei Zhou (Nanjing Medical University, Nanjing, China) for kindly provide JWA plasmid and JWA primary antibody. Dr. Lou (Mayo Clinic, MN, USA) for providing pIRES-Flag-topoisomerase IIα plasmid. This work was supported by National Natural Science Foundation of China (81472977), Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (HG11–4302), Foundation from Priority Academic Program Development of Jiangsu Higher Education Institutions and Foundation from Six Big Talent Peak of Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Y.L. and Q.F. designed the study. Y.L., X.S. and X.W. carried out the experiments. A.L., J.Z., P.J. and P.W. supported and advised the research. Y.L. and Q.F. wrote and revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, Y., Shen, X., Wang, X. et al. EGCG regulates the cross-talk between JWA and topoisomerase IIα in non-small-cell lung cancer (NSCLC) cells. Sci Rep 5, 11009 (2015). https://doi.org/10.1038/srep11009
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep11009
This article is cited by
-
Antileukemic Scalarane Sesterterpenoids and Meroditerpenoid from Carteriospongia (Phyllospongia) sp., Induce Apoptosis via Dual Inhibitory Effects on Topoisomerase II and Hsp90
Scientific Reports (2016)
-
Inhibitory effect of (−)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth
Infectious Agents and Cancer (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.