Abstract
Liver transplantation is the treatment of choice for chronic liver failure, although it is complicated by donor shortage, surgery-related complications and immunological rejection. Cell transplantation is an alternative, minimally invasive treatment option with potentially fewer complications. We used human palatine tonsil as a novel source of mesenchymal stem cells (T-MSCs) and examined their ability to differentiate into hepatocyte-like cells in vivo and in vitro. Carbon tetrachloride (CCl4) mouse model was used to investigate the ability of T-MSCs to home to the site of liver injury. T-MSCs were only detected in the damaged liver, suggesting that they are disease-responsive. Differentiation of T-MSCs into hepatocyte-like cells was confirmed in vitro as determined by expression of hepatocyte markers. Next, we showed resolution of liver fibrosis by T-MSCs via reduction of TGF-β expression and collagen deposition in the liver. We hypothesized that autophagy activation was a possible mechanism for T-MSC-mediated liver recovery. In this report, we demonstrate for the first time that T-MSCs can differentiate into hepatocyte-like cells and ameliorate liver fibrosis via autophagy activation and down-regulation of TGF-β. These findings suggest that T-MSCs could be used as a novel source for stem cell therapy targeting liver diseases.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) can be differentiated into a variety of tissue-specific lineages and have been demonstrated to possess immune regulatory function1,2,3. Bone marrow (BM) and adipose tissue are the major sources of MSCs that have been identified so far; however, their use in clinical application is limited due to the low cell yields and the invasive procedures required to isolate these cells4. Therefore, it would be desirable to find alternative and non-invasive sources of MSCs. Tonsillectomy is the most common operative procedure for tonsil hyperplasia and inflammation and the removed tissue is usually discarded5,6. Along with others, we have isolated MSCs from palatine tonsils (T-MSCs) and shown that they undergo mesodermal differentiation and possess immunomodulatory effects7,8,9. T-MSCs have been proposed as an alternative source of adult stem cells given that they are readily available as surgically removed “waste tissue”.
Allogeneic liver transplantation is the only effective treatment available for liver failure; however, donor organs are in short supply10,11. Although liver-derived hepatocyte transplantation is a potential alternative to organ transplantation, this method still relies upon the availability of appropriate donor tissue. Therefore, non-organ sources of hepatocytes are being explored for use in cell therapies, with one possible source being adult stem cells. It has been reported that BM-MSC transplantation is effective for ameliorating liver injury in a mouse model, which appears to be mediated by the differentiation of BM-MSCs into hepatocyte-like cells12,13. However, the underlying mechanisms of action of MSCs are not well understood.
A major characteristic of hepatic fibrosis is excessive accumulation of extracellular matrix (ECM) proteins and activation of hepatic stellate cells (HSCs). Dysregulation in ECM turnover due to the activation of HSCs has been observed in fibrotic progression14,15. Activated HSCs produce collagens as well as tissue inhibitors of metalloproteinases (TIMPs 1 and 2) that prevent collagen degradation16,17, thus promoting the progression of liver fibrosis. Among many factors that drive fibrosis, transforming growth factor-β (TGF-β) has been recognized as a major mediator that increases collagen expression and activates HSCs18,19,20,21. Recently, autophagy, a process by which cells degrade and recycle proteins, has been suggested to be involved in fibrosis by modulating TGF-β expression22,23.
In this study, we examined the effects of T-MSCs in an acute liver injury. We used carbon tetrachloride (CCl4)-induced liver injury in a mouse model because CCl4 specifically targets the liver by producing a free radical (CCl3), leading to lipid peroxidation, hydropic degeneration, steatosis and hepatocellular zone 3 necrosis24,25. Herein, we showed that T-MSCs have a regenerative effect by migrating to the site of liver injury and differentiating into hepatocyte-like cells. We also demonstrated that T-MSCs exert this effect by down-regulating TGF-β expression through autophagy activation. Therefore, our findings provide the first evidence of the therapeutic potential of human T-MSCs for the treatment of liver fibrosis.
Results
Phenotype and differentiation potential of T-MSCs
T-MSCs proliferated as spindle-shaped adherent cells when examined after 3 days of culture (day 3) (Figure 1A). Staining for surface markers revealed that T-MSCs were negative for hematopoietic cell markers (CD14, CD34 and CD45) and positive for primitive cell markers (CD73, CD90 and CD105) at day 3 (Figure 1B). We also investigated the potential of T-MSCs to differentiate into cells of mesodermal origin by culturing them in differentiation culture medium for 3 weeks. Adipogenic differentiation was evaluated by lipid droplet formation, chondrogenic differentiation by sulfated proteoglycan staining and osteogenic differentiation by matrix mineralization. Results showed a multipotency of T-MSCs (Figure 1C).
Phenotype and differentiation potential of tonsil-derived mesenchymal stem cells (T-MSCs) in culture.
Morphological appearance of T-MSCs at day 3 (A). Flow cytometry analysis of cell surface markers of T-MSCs (B). Representative images of adipocyte, chondrocyte and osteocyte differentiation of T-MSCs cultured in the growth or differentiation medium (C).
T-MSCs selectively migrated into the CCl4-injured liver
Following an infusion into untreated (sham) and CCl4-treated mice, we monitored the distribution of transplanted T-MSCs using a PKH26, a fluorescent cell marker26. We observed selective recruitment of T-MSCs into the CCl4-injured liver (4.7 ± 3.0% in sham versus 32.0 ± 3.3% in CCl4 mice) using flow cytometry. Infused cells were not detected in BM (11.0 ± 1.8 in sham versus 11.2 ± 2.6 in CCl4 mice) or spleen (8.9 ± 1.8 in sham versus 9.5 ± 4.5 in CCl4 mice) (Figure 2A). The increase in the number of migrated T-MSCs in the liver of CCl4-treated mice was statistically significant compared to the liver of the control mice (P < 0.001) (Figure 2B). Recruitment of T-MSCs into the liver was also examined by immunofluorescence (IF) and again showed that T-MSCs only migrated into the site of injury (Figure 2C). Next, we examined the changes in the number of T-MSCs present in the damaged liver on days 3, 7, 10 and 17. Results showed that the homing of T-MSCs to the site of injury was at its greatest 1 week after infusion and then gradually declined (Figure 2D). Thereafter, we decided to analyze the data at 7 days post-infusion.
In vivo localization of T-MSCs in CCl4-injured liver.
Representative flow cytometry histograms of PKH-positive cells from five different mice; T-MSCs in bone marrow (BM), liver and spleen at 7 days post-infusion (A). Statistical analysis of the percentage of PKH-positive cells in BM, liver and spleen by flow cytometry (B). Localization of T-MSCs in the CCl4-injected mouse liver using IF at day 7 post-infusion (C). Changes in the number of T-MSCs present at days 3, 7, 10 and 17 post-infusion and the quantification of PKH-positive area per field of 100 DAPI-labeled cells (average of 7–12 fields) (D). The data are expressed as the means ± SEM and data were analyzed by one-way or two-way ANOVA (*P < 0.5, ***P < 0.001).
T-MSCs recruited into the injured liver differentiated into hepatocyte-like cells
In order to identify whether T-MSCs recruited into the injured liver differentiated into hepatocyte-like cells, we stained mouse liver samples with albumin and HNF-4α. As shown in Figure 3A, there was an increase in the numbers of albumin and HNF-4α positive cells at 7 days post-infusion, which suggested that T-MSCs recruited into the CCl4-injured liver differentiated into hepatocyte-like cells. To confirm this possibility in vitro, we cultured T-MSCs in differentiation medium containing insulin-like growth factor (IGF), hepatocyte growth factor (HGF), dexamethasone and oncostatin M (OSM) to induce the commitment of T-MSCs to the hepatic lineage. After 3 weeks of culture in differentiation medium, expression of albumin and HNF-4α was examined using IF staining. Results confirmed the differentiation of T-MSCs into hepatocyte-like cells (Figure 3B).
CCl4-induced liver fibrosis was ameliorated by T-MSCs injection
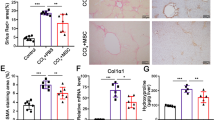
To assess whether recruited T-MSCs could ameliorate liver damage, we analyzed liver histology of CCl4-injured mice after infusion with or without T-MSCs. Using hematoxylin and eosin (H&E) staining, we observed that necrosis due to CCl4 treatment was reversed by T-MSCs infusion (Figure 4A). We also measured liver fibrosis using Sirius Red staining and found reduced collagen deposition around the central vein in the livers of CCl4–treated mice after T-MSCs infusion (Figure 4B).
The anti-fibrotic effect of T-MSCs is due to autophagy activation and reduction of TGF-β expression
Autophagy was examined in order to identify the mechanism of action of T-MSCs on liver regeneration. We stained liver tissue for the detection of LC3, beclin-1 and ubiquitin, showing that these autophagy-related proteins were expressed in T-MSCs and parenchymal cells from the CCl4-injured mice (Figure 5A). Next, we examined the expression of TGF-β, which plays a pivotal role in the progression of fibrosis. We found that the increased TGF-β expression in the CCl4-treated group was not observed in the CCl4/T-MSCs-treated group. Furthermore, the effect of T-MSCs on TGF-β down-regulation was blocked by treatment with the autophagy inhibitor, bafilomycin A1 (Figure 5B).
Autophagy induced by T-MSCs reduced TGF-β and type I collagen expression in the CCl4-damaged liver.
Confocal images of LC3, beclin-1 and ubiquitin expression in liver of control, CCl4-treated and CCl4/T-MSC-treated mice. Autophagy detected in parenchymal cells is indicated using arrows and in T-MSCs using arrow heads (A). Bafilomycin A1 (Baf) was injected with T-MSCs to inhibit autophagy. Expression of TGF-β (B) and type I collagen (C) were examined in mouse liver using IHC and IF, respectively.
Given that TGF-β signaling induces collagen synthesis, we examined type I collagen deposition in liver tissue using IF. Data showed that increased collagen I in the liver of the CCl4-injured group was reduced by T-MSCs transplantation. Autophagy inhibition again neutralized the effect of T-MSCs (Figure 5C).
Discussion
In this report, we showed the effects of T-MSCs on liver regeneration. First, we demonstrated the selective recruitment of T-MSCs into the injured liver and their differentiation into albumin-expressing, hepatocyte-like cells. Second, we observed the reduction of liver fibrosis following T-MSCs infusion. Our findings indicated that the mechanism of action of T-MSCs in resolution of acute liver damage is via an accumulation of autophagy-related proteins and inhibition of TGF-β.
Previously, we have demonstrated the recruitment of T-MSCs into damaged organs using a graft-versus-host disease (GVHD) mouse model27. In that study, we showed that the MIP-2/CXCR2 axis plays a role in the pathogenesis of GVHD by recruiting T lymphocytes into the GVHD target organs28. Given that T-MSCs secrete large amounts of CXCR2 ligand proteins such as IL-8, CXCL1 and CXCL5 (manuscript in preparation), CXCR2 may participate in the homing of T-MSCs into the injured liver. It is also suggested that HGF/c-Met signaling may be involved in the recruitment of MSCs into the damaged liver29.
Interestingly, the localization of T-MSCs in the damaged liver reached its peak at 7 days post-injection and then gradually decreased. It is possible that T-MSCs migrated to other organs or died after day 7. In addition, T-MSCs infused into normal mice were not detected in the organs that were examined, suggesting that they may have been cleared or migrated to other organs that we did not evaluate. It was reported that BM-MSCs migrated into the salivary gland when infused into the normal and salivary gland-damaged mice30. While we did not observe the localization of T-MSCs into the liver of non-injured mice, this could be due to differences in chemokine receptor profiles between stem cell populations. Therefore, it remains to be elucidated whether the infused T-MSCs migrated to other sites in the normal or CCl4-injured mice after day 7, or were simply cleared. Additionally, it will be important to determine the chemokine receptor profiles of T-MSCs, as well as their longevity in vivo.
Regarding the fate of the injected cells, we previously reported that BM-derived MSCs could differentiate into hepatocyte-like cells in vitro as well as in vivo31. Furthermore, others have demonstrated the expression of human type albumin in the host liver as an indicator of functional restoration of damaged liver after xenotransplantation32. Therefore, we investigated whether injected T-MSCs could differentiate into albumin-expressing hepatocyte-like cells. Following incubation in differentiation medium containing IGF, HGF, OSM and dexamethasone31,33, T-MSCs were shown to differentiate into hepatocyte-like cells. Expression of the hepatic parenchymal markers albumin and HNF-4α31,34 showed that our T-MSC cultures contained functional hepatocytes.
Hepatic fibrosis is a progressive pathological process involving multicellular and molecular events that ultimately lead to the deposition of excess ECM proteins such as collagens35. This study evaluated fibrosis using Sirius Red staining and IF for type I collagen. We found that collagen accumulation was significantly decreased following T-MSCs transplantation. We also demonstrated that the expression of TGF-β was down-regulated by T-MSC injection. These findings provide evidence that T-MSCs ameliorated liver fibrosis in this mouse model. It is generally accepted that HSCs are central to the process of fibrosis and are a major source of ECM components36,37. Upon activation, HSCs attain a myofibroblastic phenotype and produce ECM proteins, including enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs). Therefore, it will be important to determine whether MSCs interact with HSCs directly or indirectly and whether their interaction is involved in the resolution of liver fibrosis.
The potential use of stem cell therapy for the treatment of liver fibrosis is promising and its role in tissue regeneration has been extensively studied9,29,38. It has been suggested that autophagy may play a role in the regenerative potential of stem cell therapy9,39. Jung et al. reported that the mechanism by which human placenta-derived MSCs promote tissue regeneration may involve HIF-alpha and autophagy, with autophagy being induced in CCl4-damaged hepatic cells in a hypoxia-dependent manner9. Autophagy activation appears to be involved in T-MSC-mediated tissue regeneration as well, as shown by an increase in LC3, beclin-1 and ubiquitin expression. Inhibition of autophagy blocked the effects of T-MSCs on down-regulation of TGF-β and type I collagen expression. These results corroborate other studies that have shown the involvement of autophagy in the regulation of TGF-β expression and type I collagen synthesis22,23.
Our results indicate that T-MSCs can be a novel source of adult stem cells. T-MSCs possess the advantage over BM-MSCs or Ad-MSCs in that they can be easily obtained from “waste” tissue. However, more detailed analyses are required before T-MSCs can be developed as an alternative source for stem cell therapy, such as testing the efficacy of T-MSCs compared with other sources of adult stem cells for the treatment of disease.
In summary, we have shown that T-MSCs home into the CCl4-damaged liver and differentiate into hepatocyte-like cells. We have also demonstrated that T-MSCs can repair liver injury induced by CCl4 via autophagy activation and inhibition of TGF-β expression. This study would be the first to suggest that T-MSCs could be used in stem cell therapy as an alternative treatment for liver fibrosis.
Methods
Ethics statement and human samples
Human tonsils were obtained from tonsillectomies performed in the Department of Otorhinolaryngology, Head and Neck Surgery at Ewha Womans University Mok-Dong Hospital. The protocol was approved by the research ethics committees of Ewha Womans University Mok-Dong Hospital (IRB #ECT11-53-02). Written informed consent was obtained from all patients.
Experiments and procedures were approved by the Animal Ethics Committee at Ewha Womans University College of Medicine (ESM #11-0185) and all experiments were performed in accordance with the approved guidelines and regulations. Six- to eight-week-old female C57BL/6 mice (Oriental Bio, Seongnam, Korea) were housed at 21–23°C and 51%–54% humidity with a 12-hr light/dark cycle and supplied with food and water ad libitum.
Isolation of T-MSCs and cell culture
Tonsils were washed three times with phosphate-buffered saline (PBS) in 50 ml conical tubes, cut into ~5 mm pieces and incubated with collagenase type I (210 U/mL; Gibco BRL, Carlsbad, CA, USA) and DNase I (10 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) in 10 mL Dulbecco's Modified Eagle's Medium (DMEM, Welgene, Daegu, Korea) for 30 min at 37°C with stirring.
Cells were harvested by filtration through a cell strainer (pore size 70 μm; SPL, Pocheon, Korea) and washed twice by centrifugation (300 × g for 3 min at room temperature); cell pellets were resuspended with DMEM containing 20% heat-inactivated fetal bovine serum (FBS) for the first wash and with DMEM containing 10% FBS for the second. Mononuclear cells (MNCs) were isolated by centrifugation (300 × g for 30 min at room temperature) through a Ficoll-PaqueTM PREMIUM gradient (GE Healthcare, Pittsburgh, PA, USA). MNCs were plated at a density of 1 × 108 cells per T-150 flask in DMEM supplemented with 10% FBS, 100 μg/mL streptomycin and 100 U/mL penicillin (DMEM growth medium). Non-adherent cells were removed after 48 hrs by gentle pipetting and washing with PBS and the remaining adherent cells were cultured in DMEM growth medium and sub-cultured twice per week.
Mesodermal differentiation of T-MSCs
Adipogenic, chondrogenic and osteogenic differentiation was induced by culturing T-MSCs for 3 weeks in appropriate culture medium purchased from Invitrogen. Verification of cell populations was carried out by staining 3-week culture samples. T-MSCs cultured with adipogenic medium were fixed in 4% PFA and stained with Oil Red O staining solution for 10 min at room temperature. After inducing chondrogenic differentiation, T-MSCs were fixed in 4% PFA and cartilage formation was examined by incubating cells with Alcian blue staining solution overnight at room temperature in the dark. T-MSCs that underwent osteogenic differentiation were fixed with 60% isopropyl alcohol then stained with Alizarin Red S solution (pH 4.2) for 3 min at room temperature. For visualization, cells were examined under a phase-contrast microscope.
Cell labeling with PKH26
After 3–5 passages, T–MSCs were labeled with the PKH26 MINI kit (Sigma-Aldrich) according to the manufacture's protocol. Briefly, cell suspension (1 × 107 cells/mL) was added to the dye solution (final concentration of 2 × 10−6 M of PKH26) and immediately mixed by pipetting. After incubating for 5 min at room temperature, an equal volume of FBS was added and cells were allowed to incubate for 1 min. Cells were pelleted by centrifugation for 10 min (300 × g at room temperature), washed three times by resuspension in the same volume of DMEM growth medium, followed by final resuspension in 2 mL of PBS for mouse injection.
Flow cytometry
T-MSCs were incubated with the following primary antibodies: FITC-conjugated anti-CD45 (2D1, mouse IgG1; BD Biosciences, Franklin Lakes, NJ, USA), PE-conjugated anti-CD14 (MφP9, mouse IgG2b; BD Biosciences), FITC-conjugated anti-human CD34 (BIRMAK3, mouse IgG1; IBGRL, Bristol, UK), PE-conjugated anti-human CD73 (AD2, mouse IgG1; BioLegend, San Diego, CA, USA), FITC-conjugated anti-human CD90 (5E10, mouse IgG1; BD Biosciences) and FITC-conjugated anti-human CD105 (266, mouse IgG1; BD Biosciences).
For PKH-positive cells, livers and spleens were collected from mice on day 7 after cell transfusion, cut into 1 cm3 pieces, rinsed with 5 mL PBS, chopped with scissors and incubated in DMEM containing 100 μL/mL of collagenase D (Roche, Indianapolis, IN, USA) for 20 min at 37°C. Cell suspensions were collected and filtered through a cell strainer after the cells were centrifuged and resuspended twice with DMEM containing 10% FBS. Surface phenotype markers and PKH-positive cells were analyzed on a flow cytometer (FACSCalibur, BD Biosciences) and Cell Quest software (BD Biosciences).
Liver injury model and cell transfusion
Acute liver injury was induced using CCl4 (Sigma-Aldrich). C57BL/6 mice were randomly divided into two groups: no treatment and CCl4-injured; each group contained 5 mice. Liver-injuries were induced by intraperitoneal injection of CCl4 (10% solution in olive oil, 10 μL/g of body weight) on 2 consecutive days. T-MSCs were infused (2 × 106 per mouse) via tail vein injection on the following day and mice were sacrificed after 7 days post-infusion.
Immunofluorescence staining
Liver tissues were cryopreserved in optimal cutting temperature medium (OTC, TissueTek, SakuraAmericas, Torrence, CA, USA). Frozen tissue sections (5 μm) were collected on glass slides and fixed with 100% methanol for 10 min at 4°C, then washed in PBS for 10 min at room temperature. For in vitro differentiated cells, cells were moved to Lab-TekTMII-CC2TM chamber slide (Nalgene Nunc International, Rochester, NY, USA) on day 22 and incubated overnight at 37°C, followed by fixation with 4% PFA for 10 min at 4°C. Slides were rinsed three times with PBS for 5 min each.
Paraffin-embedded sections were de-paraffinized in xylene for 10 min and dehydrated in 100%, 95%, 90%, 80% and 75% ethanol, 3 min in each. Slides were then washed with PBS for 7 min. Enzyme-induced antigen retrieval was performed using 1× PBS containing 0.1% Trypsin for 30 min at 37°C. Slides were fixed with ice-cold methanol for 10 min at room temperature and washed twice with ice-cold PBS. PBS containing 0.25% Triton X-100 was used as permeabilization solution for intracellular staining. After blocking with 1% bovine serum albumin (BSA) in 0.02% Tween-20 in PBS (PBST), slides were incubated in a humidified chamber for 1 hr at room temperature with the following primary antibodies in 1% BSA in PBST: anti-albumin (1:100, Abcam; Cambridge, MA, USA), anti-HNF-4α (1:50, Santa Cruz Biotechnology; Dallas, TX, USA), anti-LC3 (1:50, Proteintech; Chicago, IL, USA), anti-beclin-1 (1:50, Proteintech), anti-ubiquitin (1:25, Cell Signaling Technology; Danvers, MA, USA), anti-TGF-β (1:100, Abcam) and anti-collagen I (1:500, Abcam). Slides were then washed three times in PBS for 5 min and incubated with secondary antibodies in 1% BSA in PBST: FITC-conjugated anti-rabbit IgG (1:2000, Abcam), FITC-conjugated anti-mouse IgG (1:1000, Abcam) and Cy3-conjucated anti-mouse IgG (1:1000, Abcam) for 1 hr at room temperature in the dark. Following three washes in PBS for 5 min in dark, slides were mounted using DAPI (4; 6-diamidino-2-phenylindole) mounting solution (Vector Laboratories, Youngstown, OH, USA) according to the manufacturer's instructions. Immunofluorescence was detected by confocal microscopy (LSM 5 Pascal Microscope, Carl Zeiss, Oberkochen, Germany). Control slides were incubated without primary antibodies.
H&E and Sirius Red staining
After fixation with 4% paraformaldehyde solution in PBS, paraffin embedded liver tissue sections (5 μm in thickness) were stained with H&E for histological analysis and Sirius Red (Sigma-Aldrich) staining according to standard techniques for identifying collagen deposition.
Hepatocyte differentiation in vitro
After passaging T-MSCs three times, hepatocyte differentiation was induced as follows: 106 T-MSCs were cultured for 7 days on a 100-mm culture dish in DMEM containing 5% FBS, 100 μg/mL streptomycin, 100 U/mL penicillin, 25 ng/mL IGF (R&D Systems Minneapolis, MN, USA), 25 ng/mL HGF (R&D Systems) and 10−7 M dexamethasone (Sigma-Aldrich), followed by 14 days in the same medium supplemented with OSM (10 ng/mL, R&D Systems). Culture medium was changed twice a week until day 21. Control cells were cultured without IGF, HGF, dexamethasone, or OSM for 21 days.
Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). One-way or two-way ANOVA was applied for group analysis and Student's t-test was used to identify statistically significant differences in staining (at P < 0.05). All statistical analyses were performed using GraphPad Prism Software (GraphPad Software Inc., San Diego, CA, USA).
References
Salem, H. K. & Thiemermann, C. Mesenchymal stromal cells: current understanding and clinical status. Stem cells 28, 585–596 (2010).
Shi, Y. et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell research 20, 510–518 (2010).
Le Blanc, K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy 8, 559–561 (2006).
Choudhery, M. S., Badowski, M., Muise, A. & Harris, D. T. Comparison of human mesenchymal stem cells derived from adipose and cord tissue. Cytotherapy 15, 330–343 (2013).
Forsvoll, J., Kristoffersen, E. K. & Oymar, K. Incidence, clinical characteristics and outcome in Norwegian children with periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome; a population-based study. Acta paediatrica 102, 187–192 (2013).
Papouliakos, S. et al. Comparison of clinical and histopathological evaluation of tonsils in pediatric and adult patients. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies 266, 1309–1313 (2009).
Ryu, K. H. et al. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 14, 1193–1202 (2012).
Janjanin, S. et al. Human palatine tonsil: a new potential tissue source of multipotent mesenchymal progenitor cells. Arthritis research & therapy 10, R83 (2008).
Jung, J. et al. Human placenta-derived mesenchymal stem cells promote hepatic regeneration in CCl4 -injured rat liver model via increased autophagic mechanism. Stem cells 31, 1584–1596 (2013).
Kakinuma, S., Nakauchi, H. & Watanabe, M. Hepatic stem/progenitor cells and stem-cell transplantation for the treatment of liver disease. Journal of gastroenterology 44, 167–172 (2009).
Dianat, N., Steichen, C., Vallier, L., Weber, A. & Dubart-Kupperschmitt, A. Human pluripotent stem cells for modelling human liver diseases and cell therapy. Current gene therapy 13, 120–132 (2013).
Haridass, D., Narain, N. & Ott, M. Hepatocyte transplantation: waiting for stem cells. Current opinion in organ transplantation 13, 627–632 (2008).
Russo, F. P. & Parola, M. Stem cells in liver failure. Best practice & research. Clinical gastroenterology 26, 35–45 (2012).
Hemmann, S., Graf, J., Roderfeld, M. & Roeb, E. Expression of MMPs and TIMPs in liver fibrosis – a systematic review with special emphasis on anti-fibrotic strategies. Journal of hepatology 46, 955–975 (2007).
Iredale, J. P. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis 21, 427–436 (2001).
Maher, J. J. & McGuire, R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest 86, 1641–1648 (1990).
Benyon, R. C., Iredale, J. P., Goddard, S., Winwood, P. J. & Arthur, M. J. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 110, 821–831 (1996).
Hellerbrand, C., Stefanovic, B., Giordano, F., Burchardt, E. R. & Brenner, D. A. The role of TGFβ1 in initiating hepatic stellate cell activation in vivo. Journal of hepatology 30, 77–87 (1999).
Kanzler, S. et al. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol 276, G1059–1068 (1999).
Leask, A. & Abraham, D. J. TGF-β signaling and the fibrotic response. The FASEB Journal 18, 816–827 (2004).
Dooley, S. & ten Dijke, P. TGF-beta in progression of liver disease. Cell Tissue Res 347, 245–256 (2012).
Kim, S. I. et al. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-beta1. The Journal of biological chemistry 287, 11677–11688 (2012).
Ding, Y. et al. Autophagy Regulates TGF-beta Expression and Suppresses Kidney Fibrosis Induced by Unilateral Ureteral Obstruction. J Am Soc Nephrol 25, 2835–2846 (2014).
Marques, T. G. et al. Review of experimental models for inducing hepatic cirrhosis by bile duct ligation and carbon tetrachloride injection. Acta cirurgica brasileira/Sociedade Brasileira para Desenvolvimento Pesquisa em Cirurgia 27, 589–594 (2012).
Tunon, M. J., Alvarez, M., Culebras, J. M. & Gonzalez-Gallego, J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World journal of gastroenterology: WJG 15, 3086–3098 (2009).
Liu, Y. et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med 17, 1594–1601 (2011).
Joo, S. Y. et al. Bioimaging for the monitoring of the in vivo distribution of infused mesenchymal stem cells in a mouse model of the graft-versus-host reaction. Cell biology international 35, 417–421 (2011).
Cho, K. A., Woo, S. Y., Park, Y. S., Park, M. H. & Ryu, K. H. Macrophage inflammatory protein-2 (MIP-2)/CXCR2 blockade attenuates acute graft-versus-host disease while preserving graft-versus-leukemia activity. Biochem Biophys Res Commun 426, 558–564 (2012).
Liu, J., Pan, G., Liang, T. & Huang, P. HGF/c-Met Signaling Mediated Mesenchymal Stem Cell-induced Liver Recovery in Intestinal Ischemia Reperfusion Model. International journal of medical sciences 11, 626–633 (2014).
Schwarz, S. et al. Bone marrow-derived mesenchymal stem cells migrate to healthy and damaged salivary glands following stem cell infusion. Int J Oral Sci 6, 154–161 (2014).
Jung, Y. J. et al. In vitro hepatic differentiation of human umbilical cord blood and bone marrow cells. Pediatric hematology and oncology 25, 481–491 (2008).
Kakinuma, S. et al. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem cells 21, 217–227 (2003).
Dong, X. J., Zhang, H., Pan, R. L., Xiang, L. X. & Shao, J. Z. Identification of cytokines involved in hepatic differentiation of mBM-MSCs under liver-injury conditions. World journal of gastroenterology: WJG 16, 3267–3278 (2010).
Lazaro, C. A. et al. Establishment, characterization and long-term maintenance of cultures of human fetal hepatocytes. Hepatology 38, 1095–1106 (2003).
Mederacke, I. Liver fibrosis - mouse models and relevance in human liver diseases. Zeitschrift fur Gastroenterologie 51, 55–62 (2013).
Xu, R., Zhang, Z. & Wang, F. S. Liver fibrosis: mechanisms of immune-mediated liver injury. Cellular & molecular immunology 9, 296–301 (2012).
Kisseleva, T. & Brenner, D. A. The phenotypic fate and functional role for bone marrow-derived stem cells in liver fibrosis. Journal of hepatology 56, 965–972 (2012).
Cho, K. A., Woo, S. Y., Seoh, J. Y., Han, H. S. & Ryu, K. H. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell biology international 36, 1267–1274 (2012).
Song, Y. et al. Autophagy in Hepatic Fibrosis. BioMed research international 2014, 436242 (2014).
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012M3A9C6049823).
Author information
Authors and Affiliations
Contributions
M.P., H.L. and Y.Y. collected the data. M.P., Y.K. and S.W. analyzed the data. S.W., H.K. and K.R. designed the experiments. H.K. and Y.P. provided human samples. I.J. and K.R. provided the financial support. S.J., J.P. and B.J. provided the administrative support. Y.K., S.W., I.J., H.L. and K.R. prepared the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Park, M., Kim, YH., Woo, SY. et al. Tonsil-derived Mesenchymal Stem Cells Ameliorate CCl4–induced Liver Fibrosis in Mice via Autophagy Activation. Sci Rep 5, 8616 (2015). https://doi.org/10.1038/srep08616
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep08616
This article is cited by
-
Stem Cell-based Therapy Strategy for Hepatic Fibrosis by Targeting Intrahepatic Cells
Stem Cell Reviews and Reports (2022)
-
Optimization of tenocyte lineage-related factors from tonsil-derived mesenchymal stem cells using response surface methodology
Journal of Orthopaedic Surgery and Research (2020)
-
SIRT1-modified human umbilical cord mesenchymal stem cells ameliorate experimental peritoneal fibrosis by inhibiting the TGF-β/Smad3 pathway
Stem Cell Research & Therapy (2020)
-
Wound Healing Promoting Activity of Tonsil-Derived Stem Cells on 5-Fluorouracil-Induced Oral Mucositis Model
Tissue Engineering and Regenerative Medicine (2020)
-
Conditioned Medium from Tonsil-Derived Mesenchymal Stem Cells Relieves CCl4-Induced Liver Fibrosis in Mice
Tissue Engineering and Regenerative Medicine (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.