Abstract
Medullary bone is a special bone tissue forming on the endosteal surface of the medullary cavity in the bones of female birds prior to and during egg-laying to serve as a calcium reservoir for building the hard eggshell. It has also been identified in non-avian dinosaurs, where its presence is considered as a reliable indicator of a sexually mature female. Here, we reveal that multiple mandibular symphyses of the azhdarchid pterosaur Bakonydraco galaczi possess a special bone tissue that shows all microanatomical, histological and developmental characteristics of medullary bone, despite its unusual location. Its frequent occurrence in the sample renders a pathologic origin unlikely. Our findings as well as the extremely thin-shelled eggs of pterosaurs suggest that this medullary bone-like tissue probably had a non-reproductive role in these animals. Although the non-reproductive significance and the anatomical location of this medullary bone-like tissue in Bakonydraco suggest independent evolutionary appearance from dinosaurian medullary bone, a common origin and later diverging function and physiological regulation is an equally viable phylogenetic hypothesis.
Similar content being viewed by others
Introduction
Medullary bone is a non-structural type of bone tissue that, under natural conditions, develops in the haematopoietic medullary cavities of bones of female birds induced by oestrogenic and androgenic hormones during the egg-laying cycle1,2. Under experimental conditions, administration of these hormones to male birds also results in medullary bone formation3,4,5. Growing from the endosteal layers inwards to the medullar cavity and mostly being composed of woven bone6, medullary bone has no mechanical function but serves as a calcium storage that can be quickly mobilized during the calcification of the hard-shelled eggs of birds6,7,8,9. According to the phases of the egg-laying cycle, medullary bone can be characterized by rapid formation in the period of maturing follicles and subsequent rapid destruction during the calcification of the eggshell10,11,12. This fast formation-resorption cycle of medullary bone corresponds well with its microanatomy as well as histology: (i) it has large surface areas due to its highly porous, vascularized nature; (ii) its trabeculae are composed of woven bone6; (iii) the number and activity level of osteoclasts is considerably higher in medullary bone than in cortical bone13. The composition of medullary bone is similar to that of cortical and cancellous trabecular bone; however, the proportional amount of components are different with medullary bone being more calcified, having higher apatite to collagen ratio and containing more non-collagenous proteins, proteoglycans and carbohydrates in the matrix7,12,14,15.
This bone tissue type was first described in pigeons16 and later in some other species of extant birds, among others in domestic fowl, Japanese quail, duck and ostrich3,10,11,17,18,19,20,21. The amount, microanatomy and distribution of medullary bone can be different in different species of birds as well as in different phases of the reproductive cycle1,11,17,21,22. Apart from the controversial results on mice forming medullary bone-like tissues in response to unnaturally high oestrogen doses23,24, medullary bone has not been reported in any extant non-avian amniotes25,26. Recently, with the discovery of medullary bone in different non-avian dinosaurs, the occurrence of medullary bone has proven to be phylogenetically more widespread and is considered as further evidence for the close relationship between birds and theropod dinosaurs21,27,28,29. Apart from dinosaurs, there has been only one report on the presence of an extensive, endosteally derived bone structure in a single femur of the Early Cretaceous pterosaur Pterodaustro that was tentatively interpreted as medullary bone30. Since it has been found in theropod21,27,29 as well as in ornithopod27,28 dinosaurs but not in extant alligators31,32, medullary bone has been suggested to have first appeared in the ornithodiran lineage of archosaurs after its divergence from crocodilians27,32.
Here we document the presence of a remarkable bone tissue in the mandibular symphyses of Bakonydraco galaczi (Fig. 1), an azhdarchid pterosaur from the Late Cretaceous (Santonian) of Hungary33. Except for its unusual anatomical location, this tissue shows all microanatomical, histological and inferred developmental characteristics of medullary bone as described in non-avian dinosaurs.
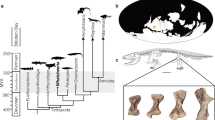
Morphology and the histological sample location of a mandibular symphysis of Bakonydraco galaczi.
(a) Silhouette drawing of the holotype lower jaw 2007.110.1 and (b) photograph of specimen 2007.111.1 (11) representing the preserved most robust part of the massive mandibular symphysis (stippled squares in (a)). Symphyses were sectioned in two cutting planes indicated by dashed lines and dashed square in (b).
Results
In all ontogenetic stages present in our sample the mandibular symphyses are completely ossified without any histological sign of former fibrous connection or interdigitating arrangement of bone in the sutural region, as seen in the variety of syndesmoses characterizing the jaw symphyses of most lizards34 and crocodiles35. This refers to a developmentally early fusion of the mandibular rami resulting in a synostotic symphysis similar to most turtles and birds35. Except for the ventrolateral and ventral symphyseal regions, an extensive erosion cavity system of highly variable spatial distribution, extent and appearance characterizes all symphyses and reflects the very dynamically changing microanatomy and microstructure of these elements. Large cavities are always present in the sagittal plane (i.e. along the expected suture line). The numerous cavities become confluent posteriorly along the longitudinal axis of the symphyses. These microanatomical features are not compatible with the hypothesis that the irregular cavity system in the jaw symphysis of Bakonydraco solely housed the mandibular branch of the trigeminal nerve or such a distinct structure as Meckel's cartilage, the latter of which has a taxon-specific but an anatomically well-defined shape, size and relative position in the mandible34,35. Hence, this cavernous system is considered as medullary cavity subdivided by thick bony columns into recesses of variable sizes (Fig. 2 and Supplementary Figs S1 and S2) that, beside nerves and blood vessels, most likely contained bone marrow (or maybe air diverticula) as well. Extensive resorption and redeposition activity within and around these recesses is evident in all specimens; however, it is markedly more intensive in specimens identified as actively growing individuals than in the two skeletally mature specimens36. The very intense remodelling results in the deposition of peculiar secondary bone tissues. Their histological appearance varies from highly porous and disorganized endosteal tissue to interwoven but highly compacted secondary bone. Transitional stages between these two extremes can be recognized among the specimens.
Symphysis specimen 2007.111.1(20) in transverse section showing the most extensive amount of medullary bone-like tissue among all investigated symphyses.
(a) Overview of a thin section under cross polarized light (inset aided by lambda wave plate) and (b) its posterior counterpart obtained from the same cut under single plane polarizers. The larger extent and merging of the recesses of the medullary cavity system is already evident in this slightly more posteriorly positioned symphyseal region. (c) Line drawing of (a) revealing the recesses of the medullary cavity system, the distribution of primary and secondary tissues and the microanatomy, relative amount and position of medullary bone-like tissue growing into the medullary cavity recesses (see also legend within the figure). (d) Close-up of the area marked by blue square in (c) under plane polarized light and (e) line drawing of the same area highlighting the microanatomy of the medullary bone-like tissue and the distribution of primary and secondary tissues as indicated by the legend. Abbreviations: Ds, dorsal symphysis surface; LAG, line of arrested growth; Ls, lateral symphysis surface; Mb, medullary bone-like tissue; Mcr, medullary cavity recess; Nf, nutritive foramen; Pb, primary bone; Vk, ventral keel.
The microanatomical location, origin, morphology and microstructure of the highly cancellous endosteal bone tissue found in the histologically least mature specimens, 2007.111.1(15) (Supplementary Fig. 2), 2007.111.1(20) (Figs 2, 3) and 2010.74.16 (Fig. 4g–i) and in the histologically adult 2010.74.18 (Fig. 4j–l) correspond with the features characterizing medullary bone of dinosaurs, including birds21,27,28,29. It is centripetally deposited on the endosteal surfaces of the cavities which are in most cases clearly separated from primary bone by a distinct resorption line and by endosteal lamellae of variable thickness (Figs 3c,e, 4h,j,k). It is highly vascularized by unusually wide canals and sinuses. The bony struts separated by these wide vascular spaces show low birefringence and contain mostly spatially unorganized, irregularly shaped lacunae apparently derived from static osteogenesis (SO)37,38,39. On the other hand, lacunae adjacent to the endosteal lamellae and those around the intact vascular canals exhibit dynamic osteogenesis-derived (DO)37,38,39 features (Figs 3b,d and 4l). Thus, this loosely organized secondary medullary tissue is composed of a combination of woven and parallel-fibred bone with the former being the dominant component of the complex and supporting its fast growing nature. Based on the architectural and structural appearance of its bony struts, different phases of the bone deposition–resorption cycle can be inferred, sometimes even in the same section. In its fully developed and early resorption stage, the intact vascular canals are organized in a laminar to plexiform to irregular architecture (Figs 2d,e and 3a-d and Supplementary Fig. 2) and may show weak lamellar infilling (Fig. 3b). The beginning of the resorption phase is characterized by the appearance of small resorption pits (Howship's lacunae) on the surface of the bony spicules (Fig. 3d) due to osteoclastic activity. Thus, in this phase, vascular canals are widened by resorption resulting in irregular sinusoid channels. In a more advanced resorption state, the degradation of this secondary bone tissue extends through its sinusoid network even further and results in larger erosion areas surrounded by scalloped bony surfaces (Fig. 3a,c,d), so that the original vascular architecture cannot be inferred anymore. The described architecture (Figs 2 and 3 and Supplementary Fig. S2) and histology (Figs 3 and 4) evidently show that this tissue is non-structural which clearly differs from both the dynamically changing structural network of load-bearing trabeculae and the irregularly arranged remnants of already resorbed cortical bone. The medullary bone-like tissue is most extensive in the posterior to middle section of 2007.111.1(20), but can also be traced to a lesser extent in the same region of 2010.74.16 and 2007.111.1(15) and in the anterior region of 2010.74.18.
Microanatomy and histology of the medullary bone-like tissue and the more compacted secondary tissues in multiple sections of 2007.111.1(20).
Note that medullary bone-like tissue is in different phases of development: areas of intact bone are seen in (a) and (b); resorption just started in (c) and (d); advanced resorption only leaves a ring of the tissue in the cavity in (f). In (c) the interwoven compact secondary tissue with large SO-lacunae is sandwiched between two recesses showing medullary bone-like tissue deposition and is separated from them by distinct resorption lines. In (e) and (f), the highly porous, unorganized secondary bone is sandwiched between the endosteal lamellae of a central cavity and the primary cortex. Image in (d) was taken under single plane polarizers; the rest of the images under crossed plane polarizers aided by lambda wave plate. Abbreviations: Dol, dynamic osteocyte lacunae; El, endosteal lamellae; Es, eroded endosteal surface; Hl, Howship lacunae; Icsb, interwoven compact secondary bone; Ipsb, interwoven porous secondary bone; Lc, lamellar compaction; Mbr, remnant of medullary bone-like tissue; rc, resorption channels and cavities; Sol, static osteocyte lacunae; Vc, vascular canal; Wbs, woven bone struts. Other abbreviations as in Fig. 2.
Medullary bone-like and other special secondary tissues found in other specimens.
(a) 2007.111.1(15); (b,c) 2010.74.10; (d–f) 2010.74.3; (g–i) 2010.74.16; and (j–l) 2010.74.18. Beside medullary bone-like tissues, the presence of highly porous and more compacted interwoven secondary bone tissues is very common among all specimens. In (i) medullary bone-like tissue is deposited on a pre-existing trabecular strut that is cut transversely in this section. Images in (b), (e), (g), (k) and (l) were taken under single plane polarizers; the rest under crossed plane polarizers aided by lambda wave plate. Abbreviations: Mz, merging zone of the confluent primary and secondary bone; Ts, trabecular strut. Other abbreviations as in Figs 2 and 3.
The secondary tissues, less reminiscent of medullary bone in their overall morphology but still considerably perforated, can be found in all specimens partially or completely filling some of the recesses or being sandwiched between the endosteal lamellae of the cavities and the primary bone (Figs 3c,e,f and 4a–d,f). The compaction level of these tissues is variable (see ‘Icsb’ and ‘Ipsb’ in Figs 3 and 4) but always higher than that of the medullary bone-like tissue with fewer but still wide vascular canals running irregularly (Figs 3e,f and 4b,c), longitudinally (Fig. 4d) or radially relative to the centre of the cavity (Fig. 4f). These secondary tissues generally show a highly interwoven structure containing lacunae of both SO- and DO-derived features (Figs 3c and 4b,d). They can merge with each other in adjacent cavities and sometimes even with the primary cortex without any sign of a distinct erosion line (Fig. 4d). Thus, their overall appearance is still strikingly different from that of the usual Haversian system and is more reminiscent of compacted coarse cancellous bone (CCCB). However, the histology and architecture of these secondary tissues show that they mostly grow into the cavities with their own, well organized vascular canal system instead of compacting irregular cavities between already existing trabeculae, as seen in CCCB. It is most likely that these structures in the Bakonydraco symphyses are identical to the unusual compacted secondary tissue found sandwiched between layers of lamellar bone in the femur V382 of Pterodaustro30.
Discussion
Apart from its non-homologous anatomical location (i.e. not in a long bone), the highly porous, secondary intramedullary bone tissue found in four symphyses of Bakonydraco galaczi (Table 1) looks identical to medullary bone as identified and described in non-avian dinosaurs21,27,28. Based on its microanatomy and microstructure, the formation principles of this tissue is most likely similar to those of fibrolamellar bone (sensu Prondvai et al.39) developing in the periosteum of juveniles of fast growing animals40,41,42,43. The marked difference is that this tissue grows centripetally within the cavities, mostly on previously resorbed bone surface. Based on its occurrence in multiple individuals in various stages of development, a pathologic origin of this tissue is highly unlikely.
Among more than 30 investigated specimens of the Early Cretaceous pterodactyloid pterosaur, Pterodaustro guinazui, Chinsamy et al.30 reported a single femur (V 382), the largest known for the taxon, that showed a considerable amount of endosteally derived bone tissue in the mid-diaphyseal region, which they proposed could represent medullary bone. However, for reasons not mentioned in their work, the authors were not entirely convinced about the true nature of this tissue30. Unfortunately, we could not properly identify the fine characteristic details on the provided images, either. Even so, related to these findings, identifying medullary bone-like tissues in the mandibular symphyses of Bakonydraco raises many important questions.
Could the nature of the described secondary tissues be the same in the two pterosaur taxa and if yes, could they be considered as medullary bone in these pterosaurs? Although found in different anatomical locations, based on the description of Chinsamy et al.30 these tissues have very similar histological characteristics in Pterodaustro and Bakonydraco, which speaks for a common tissue type. The justness of referring to them as medullary bone is harder to judge. On a pure microanatomical and microstructural basis, these medullary bone-like tissues appear to meet the criteria characterizing medullary bone. However, when considered functionally, there is substantial evidence against their expected solely reproductive role in pterosaurs for two reasons. First, in Bakonydraco primary bone histology indicates that growth rates of 2007.111.1(15), 2007.111.1(20) and 2010.74.16 were the highest among all symphyses corresponding to the earliest histologic ontogenetic stages in the sequence, with 2007.111.1(15) and 2007.111.1(20) also being the smallest specimens36 (Table 1). Skeletal immaturity alone would not exclude the sexual maturity of these specimens, as for instance, non-avian dinosaurs are believed to have started reproduction well before reaching adult body size27,44,45. However, the observation that the three, apparently fastest growing specimens exhibit medullary bone is exactly the opposite of the expected considerable slowdown of growth due to the redirection of energy from growth to reproduction44,45,46,47. Sexual maturity occurring at 53% of presumed adult size was suggested for Pterodaustro based on the histological indicators of a significant decrease in bone growth rate47. Although it is more likely that this slowdown of growth resulted from the onset of the energy consuming flight rather than that of reproduction48, marked decrease in bone growth rate is expected either way. Finally, the only unambiguously sexually mature female pterosaur found so far, a Darwinopterus specimen associated with an egg, was considered skeletally mature based on its co-ossified skull and fused postcranial elements49.
Second, pterosaurs are believed to have laid eggs with extremely thin, even leathery (“parchment-like”) shell49,50,51,52,53, similar to most oviparous non-gekkonid squamates54,55. Therefore the calcium content of eggshells and hence the calcium demand for their formation in pterosaurs must have been lower than that of the thicker, heavily calcified shells characterizing the eggs of archosaurs and turtles56,57 or even the rigid-shelled gecko eggs56. Although Chinsamy et al.30 argued that the egg of Pterodaustro was shown to have had hard shell50 and therefore this genus may have required medullary bone during egg-laying30, it is still valid that the eggshell of Pterodaustro was also extremely thin (30 μm)50. Thus, pterosaurs apparently would not have needed medullary bone for eggshell production58. Nevertheless, developing medullary bone is not a prerequisite for laying thick, hard-shelled eggs, either. Females of examined crocodilian and turtle species do not form medullary bone during their reproductive phase; however, a significant amount of endosteal structural bone is resorbed, whereas periosteal apposition is reduced in egg-laying females31,32. Not even every bird species builds up calcium reservoirs in their bones during the reproductive period. For instance, Pahl et al.22 found neither thickened bone walls, nor medullary bone development in the females of three passerine bird species before and during the laying of a clutch. Thus, the relationship between medullary bone formation and rigid eggshell production seems not as straightforward and unequivocal as it is generally claimed.
Even if not solely for reproduction, medullary bone-like tissues in these pterosaurs certainly had the capacity to serve as a calcium reservoir, but less so to have any biomechanical role. Although Fleming et al.59 reported increasing breaking strength due to medullary bone deposition in the humerus of egg laying hens, medullary bone is generally considered a non-structural bone tissue and thus biomechanical significance of similar tissues in the symphysis related to e.g., feeding habits of Bakonydraco is unlikely. As shown by the considerable size differences36 among the specimens displaying medullary bone-like tissues, the formation of this tissue is apparently size-independent further weakening a biomechanical hypothesis. On the other hand, the consistent presence of medullary bone-like tissues in the three, most actively growing specimens may suggest a role in growth dynamics. The high calcium demand during the phase of rapid skeletal growth could have required the development of such fast growing secondary bone as a special reservoir that could have been easily mobilized and thereby ensured the desired calcium supply even in periods of malnutrition or under other unfavourable conditions. This hypothesis on its own, however, is attenuated by the presence of medullary bone-like tissue in the largest, histologically adult Bakonydraco mandibular symphysis and presumably in the adult femur V 382 of Pterodaustro30. It seems more likely that high calcium turnover rate was needed for variable physiological processes in different ontogenetic stages requiring the periodic deposition of medullary bone-like tissues throughout the individual's life. These processes could have included phases of fast growth, periods of resource limitation, even migration as well as reproduction. The need for depositing a bone tissue of such high recruitment rate at any time in the skull can also relate to the lightweight construction of most postcranial bones in pterosaurs (hollow bones with extreme thin cortices58,60), which therefore may not have been able to sufficiently contribute to the general calcium metabolism without damaging their structural integrity.
The more compacted, interweaving endosteal tissues, which are present in most symphyses, most likely correspond to the unusual compacted secondary tissue described in the femur V 382 of Pterodaustro30. Although the microanatomy of these tissues looks less like that of medullary bone, for a secondary tissue unusually high vascularization and all other histological characteristics of fast formation imply that they, too, could have taken part in rapid calcium mobilization. The gradual structural transition of the medullary bone-like tissue into these interweaving endosteal tissues also speaks for a common or at least very similar, most probably physiological role. The similar tissue found in Pterodaustro was suggested to represent compacted remains of medullary bone30 as it can be observed in some laying hens61. In hens, the role of compaction and incorporation of unresorbed remains of medullary bone into the cortex is to compensate for the cortical bone loss in the reproductive phase. This is necessary because apparently not only medullary bone, but also a considerable amount of structural bone is being resorbed during egg-laying in hens61. If, indeed, the compacted secondary tissues observed in pterosaurs have similar formation principles (as their histological features indicate), resorption of the medullary bone-like tissue may have been accompanied by structural bone resorption in pterosaurs too. Such extensive bone resorption activity that necessitates fast re-deposition of structural bone resulting in these odd secondary tissues also suggests physiological periods of unusually high calcium demand. For whatever reasons, pterosaurs undoubtedly possessed a highly advanced, dynamic bone recycling system that seems to have been active, at least in Bakonydraco, throughout their life including early ontogenetic stages.
Another worthwhile hypothesis for the nature of this medullary bone-like tissue is that it represents remnants of alveolar bone. Alveolar bone develops to anchor teeth into the jaw bone, is metabolically very active62 consequently having a similar histological appearance to medullary bone (with high porosity, woven bone content and remodelling rate) and its gross anatomical location matches that of the medullary bone-like tissue in the Bakonydraco symphyses. Still, Bakonydraco was, as all azhdarchids, edentulous33, which questions the evolutionary benefits of retaining functionless alveolar bone to such extent as seen in the studied symphyses. It is still possible that alveolar bone had gained some secondary function in this pterosaur. However, in mammals alveolar bone never develops in genuinely toothless areas (e.g. diastema) because the epithelial cells of the developing dental lamina are needed to induce the cranial neural crest cell-derived ectomesenchyme to form the dental follicle which later gives rise to alveolar bone-forming osteoblasts62. This further weakens a homologous origin of alveolar bone and the medullary bone-like tissue in Bakonydraco. In addition, the presence of medullary bone-like tissues in the sagittally positioned cavities of the mandibular symphyses, including the ventralmost channels, also speaks against an alveolar bone origin. Nevertheless, the capacity of the mandible to form the medullary bone-like tissue in Bakonydraco and alveolar bone in other taxa may share some common developmental factors.
The lack of further reports on medullary bone-like tissue in other pterosaurs does not necessarily mean that the occurrence of this tissue is taxonomically restricted. Most studies identifying medullary bone in dinosaurs (including birds) focused on long bones, even though medullary bone has been reported to be present throughout the skeleton, including the skull, in laying chickens17. Although no postcranial pterosaur material can be associated with the diagnostic lower jaw of Bakonydraco galaczi with certainty63, none of the pterosaur limb bones found in the locality and sectioned so far shows such medullary bone-like tissue as do the symphyses (EP pers. obs.). This is in sharp contrast with the proportionally high frequency of this tissue in the sectioned symphyses (in four out of seven specimens) and suggests a location-specific occurrence of medullary bone-like tissues in Bakonydraco. Such a restricted, element-specific distribution can be a reason for the lack of finds among the abundant histological samples of pterosaur long bones58,60 where medullary bone-like tissues may simply be less characteristic than medullary bone is for the limb bones of dinosaurs. Possible reasons for such a distribution pattern can include biomechanical or other, yet unknown constraints on the bone wall thickness of the pterosaur postcranial skeletal elements. Therefore, it is possible that histological investigation of some cranial bones of other pterosaurs will reveal such tissues in other taxa, as well.
The ability to periodically deposit endosteally derived secondary bone, the microanatomy and histology of which indicates very high turnover rates, was unquestionably present in pterosaurs and dinosaurs, irrespective of the function and anatomical location of these tissues. Hence, it seems straightforward to conclude that their presence in pterosaurs further strengthens the hypothesis of dinosauromorphs and pterosaurs being closest sister groups63,64,65,66,67,68 (but see Bennett69,70 for contrary opinion). However, the apparently very rare occurrence of medullary bone-like tissues in the limb bones of pterosaurs and the presumed differences in their function may suggest independent evolutionary appearances of these tissues in the two clades. Alternatively, the dedicated reproductive function of medullary bone-like tissues could have evolved later on the dinosaurian lineage in which case these tissues may have common, homologous origin with diverging functions and physiological regulatory systems. A common evolutionary origin may gain some support by the findings of Cerda and Pol71 who revealed medullary-bone like tissue in the basal sauropodomorph Mussaurus patagonicus, albeit in a recent study Cerda et al.72 reconsidered it as a pathologic condition possibly originating from avian osteopetrosis. Although close-up images of the lacunar features were not provided, the described fine-scale histological characteristics based on which they argued for this tissue to be distinct from medullary bone were its high density of large osteocyte lacunae and the lamellar coating of its vascular canals72. These features, however, match the characteristics of the apparently non-pathologic medullary bone-like tissues in the Bakonydraco symphyses. In accordance with the original interpretation70, this resemblance raises the possibility that the strange tissue in Mussaurus may not be pathologic but instead shows an evolutionary earlier stage of histological appearance with unknown functional aspects.
The gradual evolutionary change hypothesis leads back to the problem of calling such tissues medullary bone in extinct animals. On one hand, if we accept that medullary bone can be identified by its microanatomical and histological features without having firm evidence of its reproductive function, as is the case in effect in all extinct taxa, then pterosaurs apparently possess medullary bone which may not be used as an ultimate indicator of sexual maturity in these animals. On the other hand, if the term medullary bone is also restricted by its reproductive function along with its hormonal regulatory system as described in birds, then similar tissues cannot be unambiguously identified as medullary bone in any extinct taxon, because we have no direct evidence of these characteristics. Although the dinosaurian nature of birds still strongly supports the reproductive role of medullary bone in non-avian dinosaurs, the general concept that medullary bone-like structures develop only in female animals being in their reproductive (egg laying) cycle, as in birds21,27,28,29 should be considered more circumspectly.
In any case, our findings strongly encourage further research on the potential alternative functions as well as evolutionary origin, distribution and significance of medullary bone-like tissues.
Methods
Along with more than fifty other specimens36, the seven mandibular symphyses of the azhdarchid pterosaur Bakonydraco galaczi studied here were found at the Iharkút vertebrate locality, in the Upper Cretaceous (Santonian) layers of the Csehbánya Formation33. All specimens belong to the Vertebrate Paleontological Collection (V PAL) of the Hungarian Natural History Museum (MTM) and are referred to only by their specimen numbers in the text. Preparation was carried out as described by Prondvai et al.36.
The seven mandibular symphyses studied here were all sectioned and histologically investigated by Prondvai et al.36 who reconstructed the relative ontogenetic sequence of the specimens with qualitative and quantitative histological methods. The reconstructed length and ontogenetic sequence of the specimens supported by most of the histological analyses in the latter study is shown in Table 1.
After recognizing medullary bone-like tissues in one of the cross sections prepared for a previous study36, additional cross and longitudinal thin sections were prepared of all specimens for the current study using the same methods (Fig. 1). Histological features of the thin sections were examined under Nikon LV 100 polarized light microscope (Nikon Corp., Tokyo, Japan). Pictures of the thin sections were taken with a QImaging MP5.0 digital microscope camera (QImaging Corp., Surrey BC., Canada) and processed with Image Pro Insight 8.0 (Media Cybernetics L.P., Maryland, USA) software.
Histological descriptions and tissue identification follow the concept of Stein and Prondvai38 and Prondvai et al.39, vascular architecture categories that of Francillon-Vieillot et al.73. The current study focuses on the secondary bone structures found in these specimens; primary tissues have already been described in detail by Prondvai et al.36. Medullary bone-like tissues are evaluated following the criteria applied for medullary bone in other fossil taxa21,27,28.
References
Schraer, H. & Hunter, S. J. The development of medullary bone: A model for osteogenesis. Comp. Biochem. Phys. A 82, 13–17 (1985).
Dacke, C. G. et al. Medullary bone and avian calcium regulation. J. Exp. Biol. 184, 63–88 (1993).
Landauer, W., Pfeiffer, C. A., Gardner, W. U. & Shaw, J. C. Blood serum and skeletal changes in two breeds of ducks receiving estrogens. Endocrinology 28, 458–464 (1941).
Miller, S. C. & Bowman, B. M. Medullary bone osteogenesis following estrogen administration to mature male Japanese quail. Dev. Biol. 87, 52–63 (1981).
Wilson, S. & Thorp, B. H. Estrogen and cancellous bone loss in the fowl. Calcif. Tissue Int. 62, 506–511 (1998).
Bonucci, E. & Gherardi, G. Histochemical and electron microscope investigations on medullary bone. Cell Tissue Res. 163, 81–97 (1975).
Ascenzi, A., Francois, C. & Bocciarelli, S. D. On the bone induced by estrogens in birds. J. Ultrastruct. R. 8, 491–505 (1963).
Müller, W. J., Schraer, R. & Schraer, H. Calcium metabolism and skeletal dynamics of laying pullets. J. Nutr. 84, 20–26 (1964).
Hurwitz, S. Calcium turnover in different bone segments of laying fowl. American J. Physiol. 208, 203–207 (1965).
Bloom, W., Bloom, M. A. & McLean, F. C. Calcification and ossification. Medullary bone changes in the reproductive cycle of female pigeons. Anat. Rec. 81, 443–475 (1941).
Bloom, M. A., Domm, L. V., Nalbandov, A. V. & Bloom, W. Medullary bone of laying chickens. Am. J. Anat. 102, 411–453 (1958).
Wang, X., Ford, B. C., Praul, C. A., Leach, J. & Roland, M. Characterization of the non-collagenous proteins in avian cortical and medullary bone. Comp. Biochem. Physiol. B 140, 665–672 (2005).
Van de Velde, J. P., Vermeiden, J., Hagen, B. J., Van Ginkel, F. C. & Prahl-Andersen, B. Histological and radiological quantification of estradiol-induced medullary bone formation and osteoclast activity in male quail. Bone 6, 391–393 (1985).
Candlish, J. K. & Holt, F. J. The proteoglycans of fowl cortical and medullary bone. Comp. Biochem. Physiol. B 40, 283–290 (1971).
Taylor, T. G., Simkiss, K. & Stringer, D. A. The skeleton: its structure and metabolism. In: Physiology and biochemistry of the domestic fowl (Freeman, F.M., ed.) 125–170 (Academic Press, New York, 1971).
Kyes, P. & Potter, T. S. Physiological marrow ossification in female pigeons. Anat. Rec. 60, 377–379 (1934).
Taylor, T. G. & Moore, J. H. Avian medullary bone. Nature 172, 504–505 (1953).
Miller, S. C. 1978 Rapid activation of the medullary bone osteoclast cell surface by parathyroid hormone. J. Cell Biol. 76, 615–618 (1953).
Miller, S. C., Bowman, B. M. & Myers, R. L. Morphological and ultrastructural aspects of the activation of avian medullary bone osteoclasts by parathyroid hormone. Anat. Rec. 208, 223–231 (1984).
Ohashi, T., Kusuhara, S. & Ishida, K. Estrogen target cells during the early stage of medullary bone osteogenesis: immunohistochemical detection of estrogen receptors in osteogenic cells of estrogen-treated male Japanese quail. Calcif. Tissue Int. 49, 124–127 (2013).
Schweitzer, M. H., Wittmeyer, J. & Horner, J. R. Gender-specific reproductive tissue in ratites and Tyrannosaurus rex. Science 308, 1456–1460 (2005).
Pahl, R., Winkler, D. W., Graveland, J. & Batterman, B. W. Songbirds do not create long-term stores of calcium in their legs prior to laying: results from high-resolution radiography. Proc. R. Soc. Lond. [Biol.] 264, 239–244 (1997).
Samuels, A., Perry, M. J. & Tobias, J. H. High dose estrogen induces de novo medullary bone formation in female mice. J. Bone Miner. Res. 14, 178–186 (1999).
Turner, R. T. Mice, estrogen and postmenopausal osteoporosis. J. Bone Miner. Res. 14, 187–191 (1999).
Edgren, R. A. A seasonal change in bone density in female musk turtles, Sternothaerus odoratus (Latreille). Comp. Biochem. Phys. 1, 213–217 (1960).
Simkiss, K. Influence of large doses of oestrogens on the structure of the bones of some reptiles. Nature 190, 1217–1217 (1961).
Lee, A. H. & Werning, S. Sexual maturity in growing dinosaurs does not fit reptilian growth models. Proc. Natl. Acad. Sci. USA 105, 582–587 (2008).
Hübner, T. R. Bone histology in Dysalotosaurus lettowvorbecki (Ornithischia: Iguanodontia) – variation, growth and implications. PLoS ONE 7, e29958 (2012).
Chinsamy, A., Chiappe, L. M., Marugán-Lobón, J., Chunling, G. & Fengjiao, Z. Gender identification of the Mesozoic bird Confuciusornis sanctus. Nat. Commun. 4, 1381 (2013).
Chinsamy, A., Codorniú, L. & Chiappe, L. M. Palaeobiological implications of the bone histology of Pterodaustro guinazui. Anat. Rec. 292, 1462–1477 (2009).
Wink, C. S. & Elsey, R. M. Changes in femoral morphology during egg laying in Alligator mississippiensis. J. Morphol. 189, 183–188 (1986).
Schweitzer, M. H., Elsey, R. M., Dacke, C. G., Horner, J. R. & Lamm, E.-T. Do egg-laying crocodilian (Alligator mississippiensis) archosaurs form medullary bone? Bone 40, 1152–1158 (2007).
Ősi, A., Weishampel, D. B. & Jianu, C.-M. First evidence of azhdarchid pterosaurs from the Late Cretaceous of Hungary. Acta Palaeontol. Pol. 50, 777–787 (2005).
Holliday, C. M., Gardner, N. M., Paesani, S. M., Douthitt, M. & Ratliff, J. L. Microanatomy of the mandibular symphysis in lizards: patterns in fiber orientation and Meckel's cartilage and their significance in cranial evolution. Anat. Rec. 293, 1350–1359 (2010).
Holliday, C. M. & Nesbitt, S. J. Morphology and diversity of the mandibular symphysis of archosauriforms. In: Anatomy, phylogeny and palaeobiology of early archosaurs and their kin. (Nesbitt, S. J., Desojo, J. B. & Irmis, R. B. eds) 555–571 (Geological Society, London, Special Publications, 379, 2013).
Prondvai, E., Bodor, E. & Ősi, A. Does morphology reflect osteohistology-based ontogeny? A case study of Late Cretaceous pterosaur jaw symphyses from Hungary reveals hidden taxonomic diversity. Paleobiology 40, 288–321 (2014).
Marotti, G., Ferretti, M., Palumbo, C. & Benincasa, M. Static and dynamic bone formation and the mechanism of collagen fibre orientation. Bone 25, 156 (1999).
Stein, K. & Prondvai, E. Rethinking the nature of fibrolamellar bone: an integrative biological revision of sauropod plexiform bone formation. Biol. Rev. Camb. Philos. Soc. 89, 24–47 (2014).
Prondvai, E., Stein, K. H. W., Ricqlès, A. d. & Cubo, J. Development-based revision of bone tissue classification: the importance of semantics for science. Biol. J. Linn. Soc. in press.
Ricqlès, A. d. Evolution of endothermy: histological evidence. Evol. Theor. 1, 51–80 (1974).
Horner, J. R., Ricqles, A. d. & Padian, K. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: growth dynamics and physiology based on an ontogenetic series of skeletal elements. J. Vertebr. Paleontol. 20, 115–129 (2000).
Ricqlès, A. d., Padian, K. & Horner, J. R. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Ann. Paleontol. 89, 67–101 (2003).
Ricqlès, A. d., Padian, K., Knoll, F. & Horner, J. R. On the origin of high growth rates in archosaurs and their ancient relatives: Complementary histological studies on Triassic archosauriforms and the problem of a “phylogenetic signal” in bone histology. Ann. Paleontol. 94, 57–76 (2008).
Erickson, G. M. Assessing dinosaur growth patterns: a microscopic revolution. Trends Ecol. Evol. 20, 677–684 (2005).
Erickson, G. M., Curry Rogers, K., Varricchio, D., Norell, M. A. & Xu, X. Growth patterns in brooding dinosaurs reveals the timing of sexual maturity in non-avian dinosaurs and genesis of the avian condition. Biol. Lett. 3, 558–561 (2007).
Chinsamy-Turan, A. The Microstructure of Dinosaur Bone (Johns Hopkins University Press, Baltimore, 2005).
Chinsamy, A., Codorniu, L. & Chiappe, L. Developmental growth patterns of the filter feeder pterosaur Pterodaustro guinazui. Biol. Lett. 4, 282–285 (2008).
Prondvai, E., Stein, K., Ősi, A. & Sander, P. M. Life history of Rhamphorhynchus inferred from bone histology and the diversity of pterosaurian growth strategies. PloS ONE 7, e31392 (2012).
Lü, J.-C. et al. An egg-adult association, gender and reproduction in pterosaurs. Science 331, 321–324 (2011).
Chiappe, L., Codorniu, L., Grellet-Tinner, G. & Rivarola, D. Argentinian unhatched pterosaur fossil: New pterosaur-egg features add to our understanding of these extinct flying reptiles. Nature 432, 571–572 (2004).
Ji, Q. et al. Pterosaur with a leathery shell. Nature 432, 572 (2004).
Grellet-Tinner, G., Wroe, S., Thompson, M. B. & Ji, Q. A note on pterosaur nesting behavior. Hist. Biol. 19, 273–277 (2007).
Unwin, D. M. & Deeming, D. C. Pterosaur eggshell structure and its implications for pterosaur reproductive biology. Zitteliana B 28, 199–207 (2008).
Grine, F. E. & Kitching, J. W. Scanning electron microscopy of early dinosaur egg shell structure: a comparison with other rigid sauropsid eggs. Scanning Micros. 1, 615–630 (1987).
Wang, X. et al. Sexually dimorphic tridimensionally preserved pterosaurs and their eggs from China. Curr. Biol. 24, 1–8 (2014).
Packard, M. J. & DeMarco, V. G. Eggshell structure and formation in eggs of oviparous reptiles. In: Egg incubation: its effects on embryonic development in birds and reptiles (Deeming, D. C. & Ferguson, M. W. J., eds) 53–69 (Cambridge Univ. Press, Cambridge, 1991).
Packard, M. J. Patterns of mobilization and deposition of calcium in embryos of oviparous, amniotic vertebrates. Isr. J. Zool. 40, 481–492 (1994).
Steel, L. The palaeohistology of pterosaur bone: an overview. Zitteliana B 28, 109–125 (2008).
Fleming, R. H., McCormack, H. A., McTeir, L. & Whitehead, C. C. Medullary bone and humeral breaking strength in laying hens. Res. Vet. Sci. 64, 63–67 (1998).
Ricqlès, A. d., Padian, K., Horner, J. R. & Francillon-Vieillot, H. Palaeohistology of the bones of pterosaurs (Reptilia: Archosauria): anatomy, ontogeny and biomechanical implications. Zool. J. Linn. Soc. 129, 349–385 (2000).
Whitehead, C. C. Overview of bone biology in the egg-laying hen. Poult. Sci. 83, 193–199 (2004).
Hall, K. B. Bones and Cartilage: Developmental and Evolutionary Biology (Elsevier Academic Press, California, 2005).
Ősi, A., Buffetaut, E. & Prondvai, E. New pterosaurian remains from the Late Cretaceous (Santonian) of Hungary (Iharkút, Csehbánya Formation). Cret. Res. 32, 456–463 (2011).
Padian, K. The origin of pterosaurs. In: Proceedings of the 3rd Symposium on Mesozoic Terrestrial Ecosystems (Reif, W.-E. & Westphal, F., eds) 163–168 (Attempto, Tübingen, 1984).
Gauthier, J. Saurischian monophyly and the origin of birds. In: The origins of birds and the evolution of flight (Padian, K, ed.) 1–55 (Mem. California Acad. Sci., San Francisco, 1986).
Sereno, P. C. Basal archosaurs: Phylogenetic relationships and functional implications. Soc. Vert. Paleontol. Mem. 2, 1–51 (1991).
Benton, M. J. Scleromochlus taylori and the origin of dinosaurs and pterosaurs. Philos. Trans. R. Soc. London B 354, 1423–1446 (1999).
Hone, D. W. E. & Benton, M. J. An evaluation of the phylogenetic relationships of the pterosaurs among archosauromorph reptiles. J. Syst. Palaeontol. 5, 465–469 (2007).
Bennett, S. C. The phylogenetic position of the Pterosauria within the Archosauromorpha. Zool. J. Lin. Soc. London 118, 261–308 (1996).
Bennett, S. C. The phylogenetic position of the Pterosauria within the Archosauromorpha re-examined. Hist. Biol. 25, 545–563 (2012).
Cerda, I. A. & Pol, D. Evidence for gender-specific reproductive tissue in a basal sauropodomorph dinosaur from the Late Triassic of Argentina. Ameghiniana 50, 11–12R (2013).
Cerda, I. A., Chinsamy, A. & Pol, D. Unusual endosteally formed bone tissue in a Patagonian basal sauropodomorph dinosaur. Anat Rec 10.1002/ar.22954 (2014).
Francillon-Vieillot, H. et al. Microstructure and mineralization of vertebrate skeletal tissues. In: Skeletal biomineralization: patterns, processes and evolutionary trends (Carter, J. E., ed.) 471–548 (Van Nostrand Reinhold, New York, 1990).
Acknowledgements
The authors are grateful to A. Ősi for access to specimens and for providing the logistic background of this research. We thank Zs. Hajdu, D. Csengődi and R.Kalmár for technical assistance. Useful discussions with K. Padian, S. Werning and A. Kellner on this topic helped improve an earlier version of the manuscript. Field and laboratory work was supported by the MTA–ELTE Lendület Program (Grant no. 95102) and the Hungarian Scientific Research Fund (OTKA T–38045, PD 73021, NF 84193).
Author information
Authors and Affiliations
Contributions
E.P. and K.S. designed the research, performed the investigation and discussed the results; E.P. performed the artwork and wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Prondvai, E., Stein, K. Medullary bone-like tissue in the mandibular symphyses of a pterosaur suggests non-reproductive significance. Sci Rep 4, 6253 (2014). https://doi.org/10.1038/srep06253
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep06253
This article is cited by
-
Systemic distribution of medullary bone in the avian skeleton: ground truthing criteria for the identification of reproductive tissues in extinct Avemetatarsalia
BMC Evolutionary Biology (2019)
-
Medullary bone in an Early Cretaceous enantiornithine bird and discussion regarding its identification in fossils
Nature Communications (2018)
-
Vascularised endosteal bone tissue in armoured sauropod dinosaurs
Scientific Reports (2016)
-
Chemistry supports the identification of gender-specific reproductive tissue in Tyrannosaurus rex
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.