Abstract
T-regulatory cells (Tregs) are a rare lymphocyte subtype that shows promise for treating infectious disease, allergy, graft-versus-host disease, autoimmunity and asthma. Clinical applications of Tregs have not been fully realized because standard methods of expansion ex vivo produce heterogeneous progeny consisting of mixed populations of CD4 + T cells. Heterogeneous progeny are risky for human clinical trials and face significant regulatory hurdles. With the goal of producing homogeneous Tregs, we developed a novel expansion protocol targeting tumor necrosis factor receptors (TNFR) on Tregs. In in vitro studies, a TNFR2 agonist was found superior to standard methods in proliferating human Tregs into a phenotypically homogeneous population consisting of 14 cell surface markers. The TNFR2 agonist-expanded Tregs also were functionally superior in suppressing a key Treg target cell, cytotoxic T-lymphocytes. Targeting the TNFR2 receptor during ex vivo expansion is a new means for producing homogeneous and potent human Tregs for clinical opportunities.
Similar content being viewed by others
Introduction
T- regulatory cells (Tregs) are a small subset of T-lymphocytes with diverse clinical applications in transplantation, allergy, infectious diseases, GVHD, autoimmunity, cancer, among others1,2,3,4,5,6,7,8,9,10. One fundamental problem stymieing their clinical development is their relative paucity: naturally occurring Tregs constitute only 1–5% of total CD4+ T cells in blood and remain largely dormant until activated. Their expansion is therefore crucial for harvesting sufficient quantities to investigate their roles in basic biology and clinical medicine11,12. Standard methods of Treg expansion13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29 are conducted ex vivo for reinfusion into patients because the expansion agents are too toxic for in vivo administration. Four expansion agents are commonly used alone or in various combinations: IL-2, anti-CD3, anti-CD3 plus anti-CD28 and rapamycin. However, those standard agents are problematic because they produce heterogeneous progeny consisting of phenotypically and functionally mixed populations of CD4+ T cells. Heterogeneous CD4+ T cell populations hold risk because they are capable of releasing pro-inflammatory cytokines and they possess cells with diverse, sometimes antagonistic functions. Heterogeneous populations are also deemed by regulatory agencies as impure and irreproducible, impeding the advance of human clinical trials. Thus a major research goal has been to find new ligands to selectively expand Tregs into homogeneous progeny.
In humans, Tregs are defined by co-expression of CD4+ and high expression of the interleukin-2 (IL-2) receptor alpha chain CD25hi. Tregs also feature inducible levels of intracellular transcription factor forkhead box P3 (FOXP3)30,31. Here we chose to focus on TNF and its receptors on Tregs. While animal studies indicate that TNF induces proliferation of Tregs, the evidence in humans, both in vivo and in vitro, is conflicting and the topic for review articles attempting to explain the contradictory literature32,33. It has been speculated that the conflicting findings could be due in part to the manufacturing problems with TNF and its tendency in culture to aggregate into different complexes, perhaps with different signaling consequences34. Also TNF has two receptors, TNFR1 and TNFR2, each of which controls different signaling pathways. Unlike TNFR1, which has ubiquitous cellular expression, TNFR2 is expressed in a more limited manner, restricted primarily to subpopulations of T cells, endothelial cells and neurons35. Research in primates suggests that TNFR2-specific ligands are likely to have minimal systemic toxicity because of TNFR2's restricted cellular distribution36. Naturally occurring Tregs appear to express TNFR2 at a higher density than TNFR137,38,39. These features might make human TNFR2 an advantageous molecular target on Tregs.
We began by examining whether soluble TNF could expand human Tregs in vitro. Once we determined that TNF could successfully expand them, we then used monoclonal antibodies to tease apart the roles of TNFR1 and TNFR2. We studied the newly screened antibodies' effects on Treg proliferation, signaling, phenotype and function by multiple in vitro assays using isolated fresh human CD4 T cells from over 500 donors. In these experiments, our purpose was to compare performance of our TNF antibodies against standard methods of Treg expansion. Once we found that one TNFR2 monoclonal antibody could expand Tregs into a homogeneous population with potent functional capacity, we sought to extend our findings to humans with a small randomized, controlled clinical trial with the TNF-inducer Bacillus Calmette-Guerin (BCG), an approved drug.
Results
Functional effects of TNF and TNFR monoclonal antibodies on Tregs
The purpose of this study was to use four standard methods for Treg expansion and examine the effects of adding TNF or TNF receptor antibodies to the culture. We sought to improve the purity and potency of the expanded Tregs, perhaps creating a preparation of cells more suitable for human trials. The four expansion protocols used as a screening tool for monoclonal antibodies were tests with human T cells with a) IL-2 expansion, b) anti-CD3 expansion, c) anti-CD3 and anti-CD28 expansion and d) anti-CD3 and anti-CD28 expansion with rapamycin, all with the absence or addition of TNF and newly screened or applied TNFR receptor monoclonal antibodies.
IL-2 is important for Treg induction and maintenance in mice4. To gain some early appreciation of the human version of this cytokine on human cultured T lymphocytes, we first cultured freshly isolated human CD4+ cells from 14 human subjects only with TNF or IL-2 for 16 hours (Fig. 1). While finding no induction of Tregs, assessed by inducible FOXP3, we observed a significant increase in Tregs after adding IL-2 with TNF. This percentage increase in the numbers of Tregs was due to greater numbers of Tregs. Co-incubation of TNF and IL-2 produced a significant increase in Tregs over IL-2 alone (Fig. 1a). By flow cytometry, TNF and IL-2 co-incubation also increased the number of CD4 + CD25hi FOXP3 cells in cultured human cells from blood (Fig. 1b).
Human CD4+ T cells cultured with TNF and/or IL-2 and measured for FOXP3 expression.
(a) In freshly isolated CD4+ cells from human blood, TNF alone does not induce FOXP3 in culture, but does induce it to higher levels when co-incubated with IL-2, compared to IL-2 alone. (b) A representative flow histogram confirms greater intracellular induction of FOXP3 in CD4 + CD25hi Tregs after co-incubation with TNF and IL-2 than with IL-2 alone. The data in (a) are from 14 subjects (left panel) and 10 subjects (right panel). Figures in flow diagrams are %. (***P < 0.001, by paired t-test).
We first explored whether both TNF receptors were needed for the TNF effect. Because TNF signals through two receptors, we studied each TNFR receptor in isolation using newly produced and commercially available monoclonal antibody ligands to TNFR1 and TNFR2. Unlike cytokines that can be difficult to manufacture and have varying solubility, antibodies have excellent solubility. In freshly isolated CD4+ cells, we examined expression levels of each TNFR in relation to CD25+ expression. TNFR1 expression on CD4+ cells, regardless of CD25+ expression levels, was unchanged using flow cytometry (Fig. 2a, middle panel), whereas TNFR2 preferentially expressed CD4+CD25hi Tregs by nearly a factor of 10 (Fig. 2a, right panel). This confirms earlier studies that TNFR2 is more densely expressed on Tregs37,38,39.
TNFR2 agonist- versus antagonist-treated Tregs and opposing effects in vitro.
(a) TNFR2 is preferentially expressed on CD4 + CD25hi T cells (b) one TNFR2 antibody induced the numbers of FOXP3, acting as an agonist and the other TNFR2 antibody suppressed the numbers of FOXP3, acting as antagonist. (c) In a signaling pathway assay, purified CD4+ cells, incubated with IL-2, the TNFR2 agonist and antagonist trigger differences in relative downstream expression of mRNA, especially in signaling proteins TRAF2, TRAF3 and apoptosis inhibitor cIAP2 that are preferentially induced by TNFR2 agonism. Data represented are means ± SEM from 4 subjects. (d) TNFR2 agonist triggers greater % increase in proliferation in samples from 6 subjects measured by flow cytometry (left panel) and with CFSE measurements (right panels) and representative results from a typical experiment are presented with CFSE measurements (right panels). The numbers in a bar represent the percentage of cells that enter division. The TNFR2 antagonist suppresses CD4+ proliferation (left panel) and inhibits expansion measured by CFSE dilution (right panel). (*P < 0.05 or **P < 0.01, by paired t-test).
Screening all available TNFR1 and TNFR2 monoclonal antibodies (mAbs) on isolated CD4+ cells sampled from fresh human blood enabled selective study of each TNF receptor, unlike studying TNF, which acts through both receptors and can have manufacturing and solubility problems stemming from the use of E. Coli and yeast systems. Although most of the newly produced and commercially available TNFR1 or TNFR2 mAbs failed to induce or suppress FOXP3+ Tregs after stimulation by presence of IL-2 for 16 hours (Supplementary Figs. 1, 2), we eventually identified two TNFR2 mAbs with significant, yet opposing, effects on FOXP3 induction (Fig. 2b). Studying freshly cultured cells from 10 subjects, one TNFR2 antibody significantly induced FOXP3 expression in CD4+ human T cells and expanded these cells in culture (which we designated the “TNFR2 agonist”), whereas the other TNFR2 monoclonal had no benefit in augmenting intracellular FOXP3 expression and with these culture conditions prevented CD4 T cell proliferation (which we designated the “TNFR2 antagonist”) (Fig. 2b). The reason for the increases in FOXP3 with the newly identified TNFR2 agonist was almost entirely secondary to expansion of absolute Treg cell numbers. There was no statistical difference between IL-2 alone and IL-2 plus TNFR2 antagonist in Treg cell numbers.
Having identified two functionally opposing TNFR2 mAbs, we measured their effects on isolated CD4+ T cells by examining downstream mRNA expression in signaling proteins specific to TNF receptor activation. After either 30 minutes or 24 hour stimulation by the TNFR2 agonist or antagonist, relative mRNA expression was significantly different. The TNFR2 agonist stimulated expression of TNF, TRAF2, TRAF3, BIRC3 (cIAP2) and FOXP3 (24 hour data shown). In contrast, the TNFR2 antagonist stimulated expression of cIAP1, but not TRAF2, TRAF3 or FOXP3 (Fig. 2c). This was not only confirmed in four human subjects (Fig. 2c), but also with 32 additional human subjects with the TNFR2 agonist and an additional 8 subjects with the TNFR2 antagonist. Distinct intracellular signaling was triggered by the newly identified antibody agonist and the antibody antagonist to TNFR2 (Fig. S2b).
The effects of the TNFR2 agonist and antagonist were also studied on purified human CD4+ T cells co-cultured with anti-CD3. When CD4+ proliferation was studied with anti-CD3 combined with the TNFR2 agonist, the highest degree of proliferation occurred. This absolute expansion and proliferation was almost exclusively (85%) due to the expansion of Tregs. In contrast, the TNFR2 antagonist suppressed CD4+ proliferation relative to the control, anti-CD3 alone and relative to anti-CD3 + TNFR2 antagonist (Fig. 2d, left-most panel). The same findings were observed after 4 days by directly measuring CD4+ proliferation by flow cytometry and measuring CD4+ proliferation by CFSE dilution (Fig. 2d, three right-most panels).
Despite high expression of TNFR2 on Tregs, some TNFR2 expression is also observable on CD4+ T cells that are not true Tregs because they only express intermediate levels of CD25, i.e., CD4+CD25mid cells. We therefore studied the impact of overnight incubation on CD25mid cell subpopulations of IL-2 alone, IL-2 and TNF, or IL-2 and TNFR2 agonist, or IL-2 and TNFR2 antagonist. We found a rise in the proportion of CD25hiFOXP3− cells similar to effector cells with IL-2 and TNF stimulation alone, or IL-2 and TNFR2 agonist alone (data not shown). However, we observed suppression with IL-2 and TNFR2 antagonist relative to the other three groups. Therefore the TNFR2 agonist and antagonist, studied by the same assay, showed opposing effects on the same CD25+FOXP3- cell population.
We separated fresh human blood to obtain pure CD4+ and CD25hi co-expressing Tregs (Fig. 3). We purified and expanded these Tregs in vitro using another standard method of Treg expansion, anti-CD3 plus anti-CD28 plus IL-2 for 16 days with or without TNF, TNFR2 antagonist, or TNFR2 agonist (Fig. 3a), then rested them overnight before counting. We added rapamycin (until day 7) because it selectively expands the highest number of Tregs with greatest capacity for suppressing CD8+ cells40,41,42,43. This process successfully produced CD4+CD25hi Tregs (Fig. 3b). We assessed Treg expansion by treatment group: standard treatment, treatment with TNF, TNFR2 agonist, or TNFR2 antagonist. The TNFR2 agonist outperformed every other group, expanding Treg numbers at least twofold higher than standard treatment or antagonist treatment (Fig. 3c). Because rapamycin is known to inhibit proliferation, we examined the effects of treatment without rapamycin, yet found similarly opposing effects between agonist versus antagonist treatment, albeit at smaller mean absolute values (Supplementary Fig. 3). The yields of expanded cells tended to be less without rapamycin, but the agonist still expanded Tregs.
TNFR2 agonist versus antagonist on expansion of Treg population.
(a) Protocol for purifying CD4 + CD25hi cells from CD4+ cells from fresh blood and expanding for 16 days by incubation in 96 well round-bottom plate (2 × 104 cells/well) with anti-CD3 and anti-CD28 antibodies, human IL-2 and rapamycin. (b) Representative CD25 and FOXP3 flow diagrams of CD4+ cells before versus after CD25hi purification and expansion, indicating purity of populations. (c) Cell counts of purified Tregs, by treatment group, reveal that TNFR2 agonist induced more expansion than any other group. (**P < 0.01, by paired t-test.) The TNFR2 antagonist suppresses expansion versus standard treatment. Data in (c) are samples from 10 subjects.
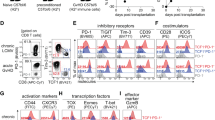
TNFR2 agonist expansion and homogeneity of Tregs
We next investigated whether Tregs, treated by TNFR2 agonist in vitro, possessed more homogeneous Treg cell surface markers than those given standard treatment or antagonist treatment. Comparing at first the phenotypes for 7 cell surface markers, all treatment groups highly expressed Treg signature markers FOXP3 and CD25 (Fig. 4a). The expression levels of FOXP3 were similar to levels before treatment (Supplementary Fig. 4, 5). Nearly 100% of expanded CD25hi Tregs in each group were positive for CTLA4, TNFR2, CD62L, Fas and negative for CD127 (Fig. 4a). Comparing the surface phenotypes for the next 7 cell surface markers, the TNFR2 agonist produced the most homogeneous cells for such markers as HLA-DR, ICOS, CD45RO, CXCR3, CCR4, CCR6 and CCR7 (Fig. 4b). Surface markers, such as HLA-DR, ICOS, CD45RO and chemokine receptors, were differentially expressed between the agonist vs. antagonist treatment (Fig. 4b,c). Similar results were observed in Tregs expanded without rapamycin (Supplementary Fig. 4). Tregs expanded by TNFR2 agonist—relative to most other comparator groups, especially the TNFR2 antagonist—yielded a surprisingly homogeneous population of cells with this phenotype: CD4+CD25highFOXP3+ CTLA4+TNFR2+CD127−CD62L+Fas+HLA-DR+CD45RO+CCR5− CCR6−CCR7−CXCR3−ICOS−. The mean fluorescence intensity (MFI), a direct measure of the average density of the protein per cell, similarly revealed that for most surface markers, the TNFR2-agonist treated cells were more homogeneous than those produced by standard treatment or TNFR2 antagonist treatment (Supplementary Fig. 6). Further investigation should determine whether these expanded cells maintain phenotypic homogeneity over time. Before treatment, Treg markers were more heterogeneous (Supplementary Fig. 5). In vitro studies of mixed Treg populations, which include CD45RO+FOXP3low T cells, produce pro-inflammatory cytokines. This particular phenotype is found in up to 50% of FOXP3+ T cells.
Homogeneity of cell surface markers on Tregs, by treatment type.
(a) All treatment groups are highly positive for Treg markers such as CD25, FOXP3, CTLA4, TNFR2, CD62L and Fas and negative for CD127. (b) TNFR2 agonist-treated Tregs almost uniformly express HLA-DR+, CD45RO+ and almost uniformly lack markers such as ICOS-, CXCR3−, CCR5−, CCR6− and CCR7−, CXCR3−. (c) Representative flow diagrams show that TNFR2 agonist-treated Tregs have greater uniformity of Treg markers than do other groups (* P < 0.05, ** P < 0.01 by t-test).
One of the most upregulated markers by TNFR2 agonist-treated Tregs was HLA-DR, which is reported to have higher suppressive activity against CD8 T+ cells, suggestive of an effector Treg44. In contrast to HLA-DR, all four chemokine receptors were strongly down-regulated. Although the lack of chemokine receptors might result in failure to migrate to the site of inflammation, another homing receptor, CD62L, which was highly expressed in all treatment groups (Fig. 4), is crucial for entering the site of pathogenic T cell presence in acute GVHD. The fact that agonist-treated Tregs were CD45RO+ and CCR7− and displayed significantly higher expression levels of Fas, measured by MFI (Supplementary Fig. 6), contributes to the view that they are activated effector Tregs.
TNFR2 agonist-treated Tregs and CD8+ suppression
One key function of Tregs, especially in autoimmunity, is to suppress the function of autoreactive cytotoxic CD8+ T cells. We assessed this functional capacity by mixing Tregs from each treatment group with CFSE-stained autologous PBMC, after having stimulated them with anti-CD3mAb and IL-2 for 4 days. Autologous CD8+ T cells, the responder cells, were tested for suppression by observing the ratios of responders to Tregs. Ratios of dilution enable study of dose-dependence. All groups of Tregs displayed suppressive function on CD8+ T cells, but the degree varied by treatment group (Fig. 5a, left panel). TNFR2 agonist-treated Tregs, for example, showed the strongest suppressive capacity at 1:1 ratio (by leaving the fewest number of CD8+ cells) and then became progressively weaker at higher ratios. However, the antagonist-treated cells displayed weaker suppressive capacity that was essentially no different from that of standard treatment. At a suppression index of 2:1, the TNFR2 agonist-treated group showed greater suppression than did the antagonist and standard treatment groups (Fig. 5a, right panel). Similar results were observed with Tregs treated without rapamycin (Supplementary Fig. 7a). The results are consistent with known phenotypes and expansion capacity of functional Tregs.
Functional assays of Tregs expanded under four treatment conditions.
(a) In a representative case, TNFR2 agonist-treated Tregs exerted stronger and dose-dependent suppression of CD8+ cell numbers, compared to other groups, at all dilutions or suppression ratios (left panel, third column). (* P < 0.05, ** P < 0.01 by t-test.) Using a suppression index of 2:1 (CD8+ Responders to Tregs), TNFR2 agonist suppression of CD8+ cells is greater than standard treatment and TNFR2 antagonist treatment (right panel). (b) TNFR2 agonist-treated Tregs produce a lower percentage of IFNγ+ cells and (c) lower numbers of T-bet+ cells after stimulation with PMA and ionomycin for 24 hours. Data in (a, right panel) are samples from 5 subjects and (b, lower panel) are from 8 subjects.
TNFR2 agonist-treated Tregs and cytokine production
We found that all treatment groups had relatively limited ability to produce intracellular IFNγ and IL-10 after PMA and ionomycin stimulation. But TNFR2 agonist and the TNF only-treated Tregs produced the lowest percentages of IFNγ+ cells (Fig. 5b, lowest left panel). The antagonist-treated Tregs showed significantly higher IFNγ production than the agonist (Fig. 5b, lower left panel). In similar experiments without rapamycin, the TNFR2 agonist-treated group not only produced lower IFNγ, but also lower IL-10 and TNF production relative to TNF or standard treatment, respectively (Supplementary Fig. 7b). Agonist-treated Tregs also showed the fewest number of TH1 Transcription Factor (T-bet) + Tregs (Fig. 5c), which is consistent with lower IFNγ production (Fig. 5c). One of the reasons for these Tregs showing high suppression capacity over CD8 T cells may be due to Tregs lacking the ability to produce IFNγ.
Clinical trial induction of human Tregs
We sought to determine in a human clinical trial whether TNF triggers Treg expansion. This trial was important because the human literature holds contradictory evidence33. Because an FDA-approved version of TNF does not exist and manufacturing of a stable human form of TNF is difficult, we administered a well-known, strong inducer of naturally secreted TNF, Bacillus Calmette Guérin (BCG). BCG is a generic vaccine already on the market for decades for treating tuberculosis and bladder cancer. In diverse animal models of autoimmunity, BCG or CFA (the equivalent of BCG but not manufactured under cGMP conditions) can induce Tregs and can eliminate autoimmunity. TNF induction is solely responsible for these beneficial effects, because they can be eliminated by anti-TNF antibodies40,41.
The small proof-of-principle, double-blind placebo-controlled clinical trial enrolled two human subjects. One subject received repeat BCG injections (1.6–3.2 × 106 cfu/injection) and one placebo subject received repeat saline, twice, 4-weeks apart. Both were monitored weekly for 20-weeks to study the pharmacokinetics of TNF and Treg induction. After each BCG injection, TNF induced Tregs in a bi-modal fashion with slightly delayed kinetics (Fig. 6a, left panels). After 20 weeks of observation, saline injections induced neither TNF nor Tregs (Fig. 6b, center panels). The total CD4+ cell counts did not change in the BCG and placebo patients other than in the percentages of CD4 + CD25hiFOXP3+ cells (data not shown). Because TNF is not the only cytokine stimulated by BCG injection, we also sought to determine whether TNF was necessary and sufficient for induction of Tregs. During routine care, a rheumatoid arthritis subject on Enbrel (etanercept), an anti-TNF antibody, was administered a BCG vaccine for protection from tuberculosis. The same tight weekly TNF and Treg monitoring revealed that Enbrel blocked both endogenous secretion of TNF and induction of Tregs (Fig. 6c, right panels). This in vivo evidence supports endogenous TNF as being obligatory for Treg induction in humans.
TNF induction of Tregs in humans and summary finding of TNFR2 agonist and antagonist activity.
(a) In a small double-blinded, placebo-controlled trial of human subjects, BCG treatment induces TNF and shortly thereafter a surge of Tregs appears in the treated subject. (b) In contrast, in the same double-blinded trial, placebo treatment induces neither TNF nor Tregs in the circulation. (c). BCG treatment of a human subject taking chronic Enbrel (etanercept), an anti-TNF antibody, reveals neither TNF nor Treg induction. This finding supports endogenous TNF as being necessary for Treg expansion in humans. (d) After expansion, the TNFR2 agonist is better than TNFR2 antagonist at proliferating and yielding more phenotypically homogeneous Tregs (CD4+ CD25hi FOXP3+ CTLA4+ TNFR2+ CD45RO+ CD62L+ CD127−, HLA-DRhi CCR5− CCR7− CXCR3− ICOS−), with higher suppression capacity for CD8+ cells and lower cytokine-producing capability. The TNF2 antagonist was able to suppress Treg expansion.
Discussion
We have developed a new method, acting through the TNF and especially the TNFR2 receptor, to obtain ex vivo an expanded and homogeneous populations of human Tregs. We identified a novel TNFR2 agonist that, combined with four standard Treg expansion protocols, IL-2, anti-CD3, anti-CD3 and anti-CD28 (with or without rapamyacin), produces phenotypically homogeneous Tregs. These homogeneous Tregs also have greater capacity to suppress cytotoxic T lymphocytes than does standard treatment. The expanded Tregs bore the near homogeneous phenotype CD4+CD25highFOXP3+ CTLA4+TNFR2+CD127−CD62L+Fas+HLA-DR+CD45RO+CCR5− CCR6−CCR7−CXCR3−ICOS−. The TNFR2-expanded Tregs did not release significant quantities of cytokines IFNγ and IL-10. We also show that Tregs could also be selectively inhibited by a different TNFR2 antibody, an antagonist. The evidence builds the case for TNFR2 as a strong molecular target with the capacity to exert bi-directional control over humans Tregs (Fig. 6d).
Tregs are expanded in ex vivo protocols because the standard agents commonly used for their expansion, including IL-2, anti-CD3 and anti-CD28, are systemically toxic. But we have also shown, through our Phase I randomized clinical trial, that a TNF-inducer, BCG, produces a surge of endogenous TNF followed by proliferation of Tregs45. BCG is a safe vaccine that has been used for more than 80 years to combat tuberculosis. This in vivo human study demonstrates the expansion of Tregs with endogenously synthesized TNF. The tantalizing prospect of eventually expanding Tregs in vivo is supported by the findings that TNFR2 has limited cellular distribution and TNFR2-specific ligands are not systemically toxic in a baboon study36.
Certain findings stand out, particularly the dose-dependent suppression of cytotoxic CD8+ T cells elicited by the TNFR2 agonist expansion of Tregs. Dose-dependency has the potential to modulate the actions of Tregs on these pivotal target cells that are lethal to other cell types. This is an attractive feature in terms of CD8+ cell targeting. Remarkably the opposing functions resulting from antibody binding to the same cell surface receptor were mediated by divergent intracellular signaling events. The TNFR2 agonist stimulated intracellular expression of important mRNAs such as TRAF2, BIRC3 (cIAP2), CHUK and FOXP3. In contrast, the TNFR2 antagonist did not stimulate intracellular expression of TRAF2, TRAF3 or FOXP3 (Fig. 2c, Fig. S2b). The normal function of the CHUK gene, part of the IKK complex, is to activate NFkB. TRAF2, also stimulated by the TNFR2 agonist, is also required for TNF-mediated activation of NFkB and the MAPK8/JNK pathway which associates with cIAP2. Therefore this signaling data is consistent with our characterization of the same receptor have opposing intracellular regulation that can be activated by two functionally opposing monoclonal antibodies.
It is remarkable how consistently the mouse literature shows TNF induction of Tregs whereas the human literature is conflicting33,46,47. Indeed TNF is a difficult cytokine to manufacture. The fact that we have identified monoclonal antibodies with preferential signaling through the TNFR2 receptor is advantageous because manufacturing antibodies is far easier than manufacturing cytokines. What is not yet known about human translational experiments using expanded Tregs in culture is their stability, durability and migratory activities leading to important clinical effects. Interestingly in our presented pilot trial and past trials, the induction of systemic TNF was followed by a brief one week long induction of Tregs that had functional properties in suppressing autoimmunity for weeks so the immunoregulation of Tregs may not directly correspond to the life of the cell (Fig. 6a)45. With properly conducted human trials, the careful dosing of these cells can be linked to the above properties48.
Taken together, this study provides the rationale for TNFR2 as a potentially attractive molecular target for in vivo and ex vivo treatment of diverse diseases in which Tregs play important roles. This single receptor has bi-directional control over Tregs, i.e., different antibody ligands can act as agonists or antagonists over one receptor. Our evidence confirms that TNFR2 is highly and preferentially expressed on Tregs with the purer CD4 + CD25hiFOXP3 phenotype expressing the highest density of TNFR247. Our findings suggest that agonist and antagonist ligands aimed at a single receptor, TNFR2, hold the potential for selectively turning on or turning off Tregs for diseases as diverse as allergy, autoimmunity, cancer, transplantation, graft versus host disease (GVHD) and infectious disease.
Methods
Human subjects and TNF induction with BCG vaccine
Over 500 individual human blood specimens served as normal blood lymphocytes for these studies through a human studies protocol approved by the Massachusetts General Hospital Human Studies Committee (MGH-2001P001379). This approval involves the obligatory informed consent for all subjects. All blood was drawn and processed within 2 hours so that the isolated human T cells were fresh and not altered by transport or storage conditions. The T cells isolated from fresh human blood were used both for the Treg assays, as well as for identification and screening of new and commercially available monoclonal antibodies to the TNF receptor.
For the interventional trial and the observational study, either one or two BCG vaccinations were used to induce TNF. Administration of two BCG vaccinations was approved by the Human Studies Committee at Massachusetts General Hospital and by the FDA (NCT00607230). Both oversight and approval committees require full informed consent from all participants. For the single BCG vaccination subject, the vaccine was administered during routine care for a travel indication in a subject that was on chronic Enbrel for her long-standing rheumatoid arthritis. The blood was drawn according to our standard protocol listed above.
For the double-blinded placebo-controlled trial with multi-dosing of BCG, one subject was injected with BCG at a dose of 1.6–3.2 × 106 cfu on two occasions and the placebo subject was injected with saline on two occasions. The BCG or saline injection was administered intradermally four-weeks apart. All blood samples were blinded and simultaneously sent to the laboratory for monitoring TNF and Treg levels.
Reagents and flow cytometry
Recombinant human TNF was purchased from Leinco Technologies (St. Louis, MO) and recombinant human IL-2 was purchased from Sigma-Aldrich (St. Louis, MO). Human monoclonal antibodies against TNFR1 and TNFR2 were from internal sources (produced through immunizations of TNFR2−/− mice and Balb/c mice with recombinant external TNFR2 membrane proteins (Recombinant Human TNF RII/TNFRSF1B, aa 24–206, R&D Systems) and external commercial vendors. External vendors included R&D Systems, Hycult- Biotechnology, BD-Pharmingen, Accurate, Abcam and Sigma for the purchase of commercially available anti-human TNFR1/2 antibodies. For the characterization of monoclonal antibodies binding to the TNFR1/2, the majority of the monoclonal antibodies bound to the receptor but neither signaled nor acted as an agonist or antagonist in functional assays (Supplementary Fig. 1). The best agonist and the best antagonist TNFR2 antibody were brought forward for study. All other antibodies to other cell surface receptors were purchased from BD-Biosciences. Intracellular staining of FOXP3 and CD152 were performed using either FOXP3 Fix/Perm Buffer set (Biolegend) or Human FOXP3 Buffer set (BD Biosciences).
Four methods of CD4+CD25+ expansion with TNF, TNFR2 monoclonal antibodies
To test various human-directed TNFR2 monoclonal antibodies for functional and expansion effects on fresh human CD4 T cells, four previously published functional assays for in vitro expansion of Tregs were tested4,16,48,49. These four expansion protocols were combined with and without the presence of various TNF receptor reagents, including TNF itself and monoclonal antibodies directed to TNFR1/2. While most of the TNFR2 directed monoclonal antibodies had no effects on Tregs, two were brought forward for intensive study because they either induced or suppressed Tregs.
First, CD4+ T cells were cultured with IL-2 and various TNF reagents. Second, CD4+ T cells were cultured with anti-CD3 antibodies and the various TNF reagents. Third, CD4+ CD25+ T were cultured with anti-CD3 and anti-CD28 and various TNF reagents and lastly, CD4+CD25+ T were cultured with anti-CD3 and anti-CD28 and rapamycin.
CD4+ T cells were isolated using Dynal CD4 Positive Isolation Kit (Invitrogen). Extraction of CD25 positive cells were subsequently performed after CD4+ isolation using Dynabeads CD25 and DETACHaBEAD CD4/CD8 (Invitrogen). After isolation, 2 × 104 cells were cultured in 96-round-bottom well plate. Dynabeads for human Treg Expander (Invitrogen) was added at a beads-to-cell ratio of 2:1. In selected wells, TNF (20 ng/ml), TNFR1 or TNFR2 mAbs (2.5 μg/ml), rapamycin (1 μM, EMD Biosciences, San Diego, CA) was added. After two days, IL-2 (200 U/ml) was added to the culture. Half of the media was changed every 2 to 3 days containing rapamycin (until day 7) and 100 U/ml of IL-2. On day 9, additional TNF or TNFR2 mAbs were supplied into the media. On day 16, cells were harvested, Dynabeads Human Treg Expander was removed, washed and rested. On the following day, cells were analyzed.
Intracellular staining
Expanded CD4+CD25+ cells were stimulated with phorbol myristate acetate (PMA) (2 ng/ml) and ionomycin (500 ng/ml) (Sigma) for 24 hours. Monensin (GolgiStop, BD Biosciences) was added for the last 4 hours of incubation. Cells were fixed and permeabilized using Human FOXP3 Buffer Set, followed by staining with fluorochrome-conjugated IFNγ and IL-10 mAbs.
mRNA isolation
Isolated CD4+ cells were incubated in the presence of IL-2 (50 U/ml) with or without TNFR2 mAbs (2.5 μg/ml). After 3 hours, cells were collected and total RNA was isolated using RNAqueous-4PCR kit (Ambion, Austin, TX). The extracted RNA was reverse transcribed using High Capacity cDNA Reverse Transcription Kit (Applied-Biosystems, Foster City, CA).
Cell proliferation and suppression assays
For CD4 proliferation experiments, CD4+ cells were stained with 1 μM carboxyfluorescein diacetate succinimidyl ester (CFSE). Cells were plated at the density of 2 × 105 cells/well in 96-well plate with anti-CD3mAbs. Four days later, cells were collected and analyzed.
For Treg suppression assay, autologous PBMCs were used as responders. PBMC were collected using Ficoll-Paque, cryopreserved at −80°C and thawed at the day before mixed with Tregs and rested overnight in RPMI 1640 and 10 U/ml IL-2. The next day, responder cells were stained with CFSE (1 μM). Responder cells (5 × 104 cells) and expanded Tregs were mixed at various ratios and stimulated with anti-CD3mAb and IL-2. After 4 days, cells were collected and analyzed.
Statistical analysis
All data analyses were performed by the paired Student t test using GraphPad Prism-5 software (GraphPad Software, La Jolla, CA). We considered two-sided p value 0.05 as significant.
References
Liyanage, U. K. et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immuno 169, 2756–2761 (2002).
Ormandy, L. A. et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 65, 2457–2464 (2005).
Ichihara, F. et al. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 9, 4404–4408 (2003).
Ochs, H. D., Gambineri, E. & Torgerson, T. R. IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immuno Res 38, 112–121 (2007).
Brunkow, M. E. et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature Genetics 27, 68–73 (2001).
McMurchy, A. N., Bushell, A., Levings, M. K. & Wood, K. J. Moving to tolerance: clinical application of T regulatory cells. Seminars in Immuno 23, 304–313 (2011).
Sehrawat, S. & Rouse, B. T. Tregs and infections: on the potential value of modifying their function. J Leukocyte Biol 90, 1079–1087 (2011).
Miyara, M. et al. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmunity Rev 10, 744–755 (2011).
Teng, M. W., Ritchie, D. S., Neeson, P. & Smyth, M. J. Biology and clinical observations of regulatory T cells in cancer immunology. Current Topics in Micro and Immuno 344, 61–95 (2011).
Baranyi, U., Gattringer, M., Valenta, R. & Wekerle, T. Cell-based therapy in allergy. Current topics in Micro and Immuno 352, 161–179 (2011).
Taylor, P. A., Lees, C. J. & Blazar, B. R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99, 3493–3499 (2002).
Wang, X. H., Lu, L. & Jiang, S. P. Regulatory T Cells: Customizing for the Clinic. Science Translational Med 3 (2011).
Brunstein, C. G. et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011).
Tresoldi, E. et al. Stability of human rapamycin-expanded CD4+CD25+ T regulatory cells. Haematologica 96, 1357–1365 (2011).
Nadig, S. N. et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med 16, 809–813 (2010).
Battaglia, M., Stabilini, A. & Tresoldi, E. Expanding human T regulatory cells with the mTOR-inhibitor rapamycin. Methods Mol Biol 821, 279–293 (2012).
Pahwa, R. et al. Isolation and expansion of human natural T regulatory cells for cellular therapy. Journal of Immuno. Methods 363, 67–79 (2010).
Hoffmann, P., Eder, R., Kunz-Schughart, L. A., Andreesen, R. & Edinger, M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood 104, 895–903 (2004).
Lin, C. H. & Hunig, T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immuno 33, 626–638 (2003).
Lan, Q. et al. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biology 4, 22–28 (2012).
Sagoo, P. et al. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Science Translational Med 83, 1–10 (2011).
Edinger, M. & Hoffmann, P. Regulatory T cells in stem cell transplantation: strategies and first clinical experiences. Current Opinion Immuno. 23, 679–684 (2011).
Trzonkowski, P. et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127-T regulatory cells. Clinical Immuno 133, 22–26 (2009).
Di Ianni, M. et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011).
Hippen, K. L. et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 3, 83ra41 (2011).
Kim, Y. C. et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood 119, 2810–2818 (2012).
Bacchetta, R. et al. Interleukin-10 Anergized Donor T Cell Infusion Improves Immune Reconstitution without Severe Graft-Versus-Host-Disease After Haploidentical Hematopoietic Stem Cell Transplantation. ASH Annual Meeting Abstracts 114, 45 (2009).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology 143, 1207–1217 e1202 (2012).
Cardenas, P. A., Huang, Y. & Ildstad, S. T. The role of pDC, recipient T(reg) and donor T(reg) in HSC engraftment: Mechanisms of facilitation. Chimerism 2, 65–70 (2011).
Strauss, L. et al. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol 178, 320–329 (2007).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Faustman, D. & Davis, M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov 9, 482–493 (2010).
Biton, J., Boissier, M. C. & Bessis, N. TNFalpha: activator or inhibitor of regulatory T cells? Joint, bone, spine: Reviews in Rheu 79, 119–123 (2012).
Petersen, C. M. et al. Bioactive human recombinant tumor necrosis factor-alpha: an unstable dimer? Eur J Immuno 19, 1887–1894 (1989).
Carpentier, I., Coornaert, B. & Beyaert, R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem 11, 2205–2212 (2004).
Welborn III, M. B. et al. A human tumor necrosis factor p75 receptor agonist stimulates in vitro T cell proliferation but does not produce inflammation or shock in the baboon. J Exp Med 184, 165–171 (1996).
Chen, X. et al. Co-expression of TNFR2 and CD25 identifies more of the functional CD4(+)FOXP3(+) regulatory T cells in human peripheral blood. European J Immuno 40, 1099–1106 (2010).
Annunziato, F. et al. Phenotype, localization and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med 196, 379–387 (2002).
Chen, X., Baumel, M., Mannel, D. N., Howard, O. M. Z. & Oppenheim, J. J. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4(+)CD25(+) T regulatory cells. J Immuno 179, 154–161 (2007).
Ryu, S., Kodoma, S., Ryu, K., Schoenfeld, D. et al. Reversal of established autoimmune diabetes by restoration of endogenous beta cell function. J Clin Invest 108, 63–72 (2001).
Rabinovitch, A., Suarez-Pinzon, W. L., Lapchak, P. H. et al. Tumor necrosis factor mediates the protection against autoimmune diabetes in BB rate. J Autoimmunity 8, 357–66 (1995).
Coenen, J. J. A., Koenen, H., van Rijssen, E., Hilbrands, L. B. & Joosten, I. Rapamycin and not cyclosporin A, preserves the highly suppressive CD27(+) subset of human CD4(+)CD25(+) regulatory T cells. Blood 107, 1018–1023 (2006).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4(+)CD25(+)FoxP3(+) regulatory T cells. Blood 105, 4743–4748 (2005).
Abraham, R. T. & Wiederrecht, G. J. Immunopharmacology of rapamycin. Annual Review of Immuno 14, 483–510 (1996).
Faustman, D. L., Wang, L., Okubo, Y., Berger, D. et al. Proof-of-concept, randomized controlled clinical trial of BCG for treatment of long term type 1 diabetes. PLOSOne 7(8), e41756. 10.1371/journal.pone.0041756 (2013).
Mijnherr, G., Prrakken, B. J. & van Wijk, F. The effect of autoimmune arthritis treatment strategies on regulatroy T-cell dynamics. Current Opinion in Rheu 25, 260–267 (2013).
Chen, X. & Oppenheim, J. J. The phenotypic and functional consequences of tumour necrosis factor receptor type 2 expression on CD4(+) FoxP3(+) regulatory T cells. Immuno 133, 426–433 (2011).
Sakaguchi, S. & Vignali, D. A. A. et al. The pasticity and stability of regulatory T cells. Nature Reviews Immun 13, 461–467 (2013).
Battaglia, M., Stabilini, A., Migliavacca, B., Horejs-Hoeck, J., Kaupper, T. & Roncarolo, M. G. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells in both healthy subjects and type 1 diabetic patients. Jounral of Immuno 177, 8338–8347 (2006).
Acknowledgements
We thank the Iacocca Foundation for the support of this program. We thank M. Davis for her editorial comments and critique of the paper.
Author information
Authors and Affiliations
Contributions
Y.O. performed, developed the methods and analyzed all experiments in this paper except the clinical trial, T.M. performed the experiments on human donors looking for Treg intracellular signaling after TNFR2 agonism or antagonism, L.W. was involved in the overall clinical trial for this paper, D.L.F. designed the clinical trial and worked with Y.O. on the design and interpretation of the Treg experiments in this paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplemental Information S1-S7
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Okubo, Y., Mera, T., Wang, L. et al. Homogeneous Expansion of Human T-Regulatory Cells Via Tumor Necrosis Factor Receptor 2. Sci Rep 3, 3153 (2013). https://doi.org/10.1038/srep03153
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03153
This article is cited by
-
Bivalent structure of a TNFR2-selective and agonistic TNF-α mutein Fc-fusion protein enhances the expansion activity of regulatory T cells
Scientific Reports (2023)
-
The second decade of anti-TNF-a therapy in clinical practice: new lessons and future directions in the COVID-19 era
Rheumatology International (2022)
-
TNFR2 antagonist and agonist: a potential therapeutics in cancer immunotherapy
Medical Oncology (2022)
-
MiR-126, IL-7, CXCR1/2 receptors, inflammation and circulating endothelial progenitor cells: The study on targets for treatment pathways in a model of subclinical cardiovascular disease (type 1 diabetes mellitus)
Journal of Translational Medicine (2021)
-
Anti-tuberculosis chemotherapy alters TNFR2 expression on CD4+ lymphocytes in both drug-sensitive and -resistant tuberculosis: however, only drug-resistant tuberculosis maintains a pro-inflammatory profile after a long time
Molecular Medicine (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.