Abstract
The turnover of phylogenetic clades across space is a fundamental biodiversity pattern that may depend on long-term evolutionary processes and that has downstream effects on other aspects of diversity including species richness and community structure. Limited niche evolution and limited dispersal are two major processes causing spatial restriction and thus turnover, of clades. We studied the determinants of clade turnover within the World's richest floristic kingdom, the Neotropics, using the palm family (Arecaceae) as a model. We show that continental-scale clade turnover is driven by a combination of limited niche evolution — with respect to temperature and soil tolerances — and limited dispersal. These findings are consistent with strong dispersal barriers within the Neotropics and the observation that some palm lineages are most diverse in certain biomes or climates. The importance of such deep-time effects suggest that palms might be slow to adapt or disperse in response to anthropogenic climate change.
Similar content being viewed by others
Introduction
Most groups of organisms are geographically restricted in their occurrence, sometimes becoming emblematic for a region, like the lemurs for Madagascar or the palms for the tropics. This turnover of phylogenetic clades in space is a fundamental biodiversity pattern that has long fascinated biogeographers1. Two major factors seem to restrict the geographic distribution of clades: limited dispersal and limited β-niche evolution (β-niche refers to the niche dimensions that determine a species' distribution in space2). By limited dispersal we mean the failure of individuals to reach certain environmentally suitable areas, whereas by limited β-niche evolution we mean the failure to evolve adaptations to certain environments. The relative importance of these two factors is largely unstudied and likely to vary among taxa, regions and biomes3. Of note, geographic turnover of clades can lead to other diversity patterns, such as variation in species richness or the functional composition and diversity of assemblages, if there is variation in species richness or morphology among clades4,5,6. The factors leading to among-clade differences in species richness have received considerable attention7, while the factors constraining those clades in space — although a crucial piece of the puzzle — have mainly been discussed for a few prominent examples8,9.
Tropical America harbours the most diverse flora of the world10 and the origin of this wealth of plant species has received intense scientific scrutiny3,11,12,13. Scenarios of Neotropical plant evolution involve effects of both dispersal barriers and ecological factors, such as biome conservatism3,14. Influential historical events include the long-time separation of South America from North and Central America and the subsequent Great American Biotic Interchange, marine incursions into the Amazon basin and the uplift of the Andes11,15. These geomorphological reorganizations caused strong changes in dispersal opportunities and habitat distribution, with abundant dispersal barriers and steep ecological gradients. Here, we provide new insights into the complex history of Neotropical plant diversification by analysing the geographic turnover of clades within a major tropical plant family, the palms (Arecaceae).
Palms are a pantropical group largely restricted to megathermal climates16 and a textbook example of tropical niche conservatism1,9. Approaching one third of the family's 2,400 species is found in the New World17, with all but one of native species being endemic. American palms are distributed between 34°Ν and 34°S (Fig. 1a), from sea level to 3600 m.a.s.l. and occur under a wide variety of environmental conditions18. It is known that the broad-scale distributions of palm species depend on both environment — mainly climate — and dispersal19. In the Americas, many palm lineages have a spatially restricted distribution (e.g. Fig. 1 b–e). Based on the distribution of palm genera, seven biogeographic “palm regions” were proposed as putative centres of diversification (Fig. 1a)18. Geographic turnover of palm clades is also reflected in strong phylogenetic clustering of palm assemblages in “botanical countries” (TDWG level 3 units)20. A previous study indicated that the turnover of larger palm clades (subfamilies) is shaped by both β-niche evolution and dispersal limitation14. The subfamilies Arecoideae and Calamoideae were most species-rich in areas with high humidity and fertile soils, indicating limited capacity for adaptation to other environments14. Species diversity within the palm subfamilies peaked in different regions after controlling for the current environment, suggesting dispersal-limited diversification around different areas of origin14. However, the numerically dominant Arecoideae appeared to be close to equilibrium with the current environment, showing little evidence for dispersal limitation at the clade level14. Since this previous study only analysed a few pre-defined clades (the subfamilies), it remains unclear to what degree American palm lineages in general are spatially restricted by limited β-niche evolution or by dispersal.
a: Distribution, species richness and biogeographic regions of palms in the Americas. Green shading indicates the species richness of palms in 1° × 1° grid cells. Stippled lines indicate the biogeographic “palm regions” suggested by A. Henderson18: (1) Mexican region, (2) Caribbean region, (3) Central American region, (4) Andean region, (5) Amazon region, (6) Central Brazilian region, (7) Atlantic coastal forest. b–e: Examples of palm lineages with a spatially restricted distribution. b: Coccothrinax (14 spp., stem age: 10 mya), c: Syagrus (30 spp., 8.5 mya), d: Ceroxylon (11 spp., 11 mya), e: Chamaedorea (77 spp., 19 mya)18,58.
Limited dispersal and limited β-niche evolution should lead to predictable relationships between phylogenetic turnover (often called “phylogenetic beta diversity”21, but see ref. 22) and environmental and spatial distances among assemblages (Table 1). Analysing these relationships has previously provided important insights into community assembly, albeit at much smaller scales than in the present study23,24,25. Applied at large spatial scales, this approach may complement insights from biogeographic analyses26 and phylogenetic assemblage structure20. At regional scales, phylogenetic turnover is often influenced by speciation (mechanisms M1 and M2 in Table 1), causing closely related species to have strongly disjunct distributions (allopatric speciation) or strongly divergent β-niches (adaptive speciation along spatio-environmental gradients)24. At larger (continental) scales this effect is likely less pronounced and phylogenetic turnover is dominated by limited β-niche evolution and/or limited dispersal (mechanisms M3 and M4 in Table 1).
The extent to which limited dispersal leads to predictable relationships between phylogenetic turnover and spatial distance should depend strongly on the environmental heterogeneity of the study region. Even spatially close locations may be separated by strong dispersal barriers (e.g. mountain ranges, seaways), whereas even distant locations may be connected by dispersal corridors (i.e. habitat similar to the locations themselves). If a correlation between phylogenetic turnover and spatial distance is indeed due to limited dispersal (M3 in Table 1), it should increase when spatial distance is weighted with the occurrence of barriers between locations. Measuring barriers is not trivial, since any given habitat (e.g. mountain peaks) may constitute a barrier for some species (e.g. lowland rainforest species), but a dispersal corridor for others (e.g. alpine species). We developed a new measure of “barrier distance” that accounts for such differences. For each pair of locations, we mapped habitat dissimilarity — relative to the habitat at the locations themselves — throughout the study region. We then quantified the minimum amount of dissimilar habitat that needs to be crossed in order to get from one location to the other (i.e. the sum of dissimilarity scores of all grid cells crossed). Deviations from a straight line were allowed if this led to a lower barrier distance value. This captures the occurrence of dispersal corridors that indirectly connect locations. Of note, this barrier distance is inherently correlated with environmental distance between locations and it can only be compared to pure spatial distance if environmental distance is controlled.
Based on previous knowledge of the geographical ecology of palms, we derived the following specific hypotheses for our study: i. Clade turnover among palm assemblages in the Americas is determined by limited dispersal (M3) and limited β-niche evolution (M4). Speciation patterns (M1 and M2) are, if at all, of minor importance at this scale, resulting in an overall positive relationship between both environmental dissimilarity and spatial distance and phylogenetic turnover. ii. The β-niche evolution effect is strongest (based on the numerical dominance of Arecoideae, see above). iii. Climate, particularly temperature extremes and seasonality, is most important for this β-niche evolution effect. iv. If an observed relationship between phylogenetic turnover and spatial distance is caused by dispersal (M3 in Table 1), this relationship should be even stronger when spatial distance is replaced with a distance measure that takes dispersal barriers into account.
Results
The turnover of clades among palm assemblages in 1 × 1° grid cells (in the following: phylogenetic turnover) was significantly positively related to (a) spatial distance, (b) a measure combining spatial distance and the presence of barriers in between the cells (“barrier distance”) and (c) the overall environmental dissimilarity among the cells integrating climate, soils, topography and remotely sensed vegetation structure (Fig. 2a–c). All assessments of significance were based on a phylogenetic null model (see Methods). Phylogenetic turnover was also significantly positively related to environmental dissimilarities that included only one aspect of the environment (i.e. temperature dissimilarity [Fig. 2d], precipitation dissimilarity, soil dissimilarity, topographic dissimilarity and vegetation dissimilarity). When using individual environmental variables (e.g. temperature seasonality, isothermality, etc. – see Supplementary Table S1) to compute dissimilarity, 20 out of the 31 single-variable dissimilarities significantly predicted phylogenetic turnover. All results of the aforementioned models can be found in Supplementary Table S1.
Relationships between phylogenetic turnover (y-axis, in mya) and different distance measures.
a: geographic (great circle) distance, b: barrier distance, an integrated measure of geographic distance and dispersal barriers, c: environmental dissimilarity based on 31 environmental variables describing climate, soil, topography and vegetation structure, d: temperature dissimilarity based on 11 temperature variables only. All distances are rescaled to a range of [0, 1]. Dashed lines are regression lines.
The largest amount of variation in phylogenetic turnover (49%) was explained by a model that included barrier distance and dissimilarity in temperature seasonality (Supplementary Table S2). Models that included barrier distance consistently outperformed (i.e. explained 1.8–7.4% more variation, median 6.6%) the corresponding models with simple geographic distance. The fraction of variation independently explained by spatial or barrier distance (Rpd) was always significantly larger than Rpd of the null model (P ≤ 0.001). The fractions of variation that were independently explained by environmental dissimilarity (Rpe) were generally smaller when barrier distance was used than when spatial distance was used, with the exception of two environmental variables that anyway showed negligible Rpe values; however, the median difference was small (0.06%). The degree to which the environmental variables co-varied with spatial/barrier distance was also highly variable; e.g. the fraction of variation explained by both environmental and barrier distance ranged from 0.2% to 25%, depending on the way environmental dissimilarity was computed. The different environmental variables also differed widely in their independent ability to explain variation in phylogenetic turnover (Rpe of 0–19% with spatial distance, 0–14% with barrier distance). Observed Rpe was not always significantly larger than Rpe of the null model (Fig. 3, Table 2). The single-variable dissimilarities of temperature seasonality, temperature annual range and isothermality consistently had the highest independent ability to explain variation in phylogenetic turnover (Table 2). Mean temperature of the coldest quarter and minimum temperature of the coldest month also performed better than any other single-variable dissimilarity. However, the individual effects of these variables cannot be separated, because they are highly intercorrelated (Spearman's |ρ| ≥ 0.7; see Supplementary Table S3). Temperature dissimilarity also explained more variation independently (Rpe) than any other category of variables (i.e. precipitation, soil, topographic, or vegetation dissimilarity) and performed even better than the measure including all environmental variables (temperature and non-temperature). Some non-temperature single-variable dissimilarities also had significant Rpe fractions, albeit with low amounts of explained variation. These included soil pH and base saturation, total exchangeable bases (only with spatial distance), precipitation of the wettest quarter and month, annual precipitation (only with spatial distance) and annual mean QSCAT, a microwave backscatter measure of vegetation structure. Soil as a group also had a significant effect (P = 0.005) when analysed together with spatial distance.
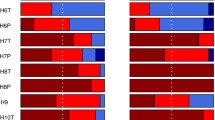
Variation in phylogenetic turnover among palm assemblages explained by environmental dissimilarity and spatial/barrier distance.
Pairs of bars correspond to different measures of environmental dissimilarity (Full = all variables PCA, Soil, Topography, Vegetation structure, Temperature and Precipitation). The upper bar in each pair is based on spatial distance and environmental dissimilarity; the lower bar on barrier distance and environmental dissimilarity. Rpd: fraction of variation explained by spatial/barrier distance only, Rmx: fraction of variation explained by either spatial/barrier distance or environmental dissimilarity, Rpe: fraction of variation explained by environmental dissimilarity only. P values indicate the probability that the Rpe fraction is not larger than the Rpe fraction of the null model (H0). Only P-values < 0.05 are shown. x-axis: fraction of variation in phylogenetic turnover.
Discussion
Our expectation (hypothesis i) that both limited β-niche evolution and dispersal limitation would contribute to the spatial turnover of palm clades was strongly supported by the results (Fig. 3). Of note, the influence of the two factors cannot be fully separated due to spatial autocorrelation in the environment; this is evidenced by the Rmx fractions of the variation partitioning (Fig. 3). However, the independent effects of limited niche evolution (Rpe) and dispersal limitation (Rpd) are sufficient to conclude that both factors have a significant and quantitatively non-negligible effect on the geographic turnover of clades.
Due to dispersal limitation, lineages often diversify within continents or islands, but fail to spread to other environmentally suitable landmasses. Within continents, dispersal limitation is more difficult to detect, because barriers are not always obvious and differ among species depending on their niche27. Pure spatial distance can serve as a crude proxy for the amount of barriers between two locations28, but this correlation is imperfect. Of note, pure spatial distance will also capture “time-for-dispersal” effects29, i.e. a limitation that is not imposed by barriers, but by the limited speed at which populations can spread across a uniformly suitable landscape. This mechanism appears to be relevant sometimes even on evolutionary timescales30. The significant effect of spatial distance on clade turnover in American palms (Rpd) can thus be interpreted as evidence for dispersal limitation on evolutionary timescales caused either by barriers or by time. We were able to gain additional insights by devising a complex measure of “barrier distance” that aimed to incorporate the species-specific suitability of areas as dispersal corridors. The fact that barrier distance explains more variation of phylogenetic turnover than spatial distance shows that the more complex measure better reflects dispersal barriers and confirms that barriers play an important role for the distribution of palm clades. This clear result is remarkable since barrier distance (i) is based on mean environmental conditions within 1 × 1° grid cells, which cannot capture the occurrence of smaller-scale dispersal barriers or corridors, (ii) ignores differences in niche breadth among species and (iii) assumes that the environment of a given grid cell is optimal for all species occurring there. In spite of these limitations, barrier distance as calculated here is clearly better suited for modelling dispersal limitation than great circle distance. Our findings likely reflect the influence of strong large-scale barriers such as seaways (e.g. separation between N and S America, isolated islands of the Carribean) and the Andes31,32,33, but potentially also of less visible environmental barriers (e.g. the savannahs separating the Brazilian Atlantic Rainforest from the Amazon).
The geographic turnover of palm clades in the Americas is clearly shaped by β-niche evolution. Our finding that assemblages in different environments tended to be phylogenetically distinct, even when spatially close and not separated by measurable barriers and that phylogenetic distinctness increases with environmental dissimilarity indicates that niche evolution is overall slow compared to the tempo of lineage diversification (Table 1: M4). Effects of limited niche evolution (sometimes called “phylogenetic niche conservatism”, but the definition of this term is controversial34,35) on clade distributions have been demonstrated previously36,37. There is also evidence showing that limited niche evolution may, via its effect on clade distributions, have shaped patterns of community structure6 or diversity4,38 in terrapins, salamanders and mammals. Our results supported the hypothesis iii that evolution of the climate niche, in particular related to temperature, had the strongest effect in American palms (Fig. 3, Table 2). It is well known that temperature is important in limiting species distributions39; this was previously documented in palms40. Our results are consistent with the fact that palms are inherently ill-suited for tolerating extreme temperatures9,41,42. Of note, while water availability probably limits the geographic ranges of American palms14,43, the evolution of those range limits does not seem to be a limiting factor for clade distributions (Fig. 3). Although scientific evidence has been lacking, the distributions of American palm species are thought to be influenced by edaphic conditions14,19. This was confirmed by our results; furthermore, edaphic range limits appeared to evolve slowly compared to the speed of clade diversification, although the effect was much weaker than for temperature (Fig. 3, Table 2). Slow evolution (“conservatism”) of edaphic niches has also been shown for other plant groups and regions44. In contrast, vegetation cover and structure either do not limit American palm ranges, or these constraints are phylogenetically labile (Fig. 3). Vegetation structure characteristics measured by remote sensing have been successfully used to model species distributions and diversity45, but probably influence palm distributions primarily at local scales19.
Our results tentatively suggest that dispersal limitation has an equal or higher influence on palm clade turnover than niche evolution, rejecting our hypothesis ii. Even if the variation in phylogenetic turnover that could be due to either of the factors (Rmx) is entirely attributed to niche evolution, the influence of both factors would be about equal (Fig. 3). The dominance of dispersal limitation is also supported by the observation that Rpd > Rpe except for the two models with highest Rpe (Supplementary Table S2). However, comparisons of the magnitude of Rpe and Rpd should be interpreted with caution since these fractions were affected by different error sources. The magnitude of Rpe is sensitive to the inclusion of relevant environmental predictors and their quality; the magnitude of Rpd depends on how well we were able to quantify the presence and strength of dispersal barriers between locations. Our results concerning the relative importance of dispersal and niche evolution rest on the assumption that these two error sources were of similar size. Of note, dispersal limitation on evolutionary timescales often requires some degree of limited niche evolution, since barriers are unsuitable areas as defined by the niche27. If niches were highly evolvable, barriers could easily be crossed by adaptation to the environment on the barrier. Any signals of dispersal limitation, such as correlations between phylogenetic turnover and spatial/barrier distance, are thus also evidence of limited niche evolution. However, this effect — mediated by dispersal — is different from the direct niche evolution effect (Table 1 M4).
In general, we consider all our estimates conservative. Uncertainty was introduced at various points, including the distribution maps, the interpolated environmental predictors, the molecular dating procedure and the artificial species level resolution. Relevant environmental predictors could also be missing46, although the set of included variables was extensive. Neither spatial distance nor our measure of “barrier distance” are perfect proxies of dispersal limitation. Any further improvement in the data sources would likely increase the amount of variation explained by dispersal limitation or niche evolution. When it becomes possible to model biotic interactions, the influence of other species on the distribution patterns and niche estimates can be added to the equation.
Besides the effects outlined above, the phylogenetic structure of assemblages may be influenced by other factors, notably speciation patterns21,24 (Table 1). At regional scales, repeated allopatric speciation across a barrier might lead to a negative correlation between phylogenetic turnover and spatial or barrier distance47. This pattern was found in a study on phylogenetic turnover among hummingbird communities in Ecuador (ca. 700 km in extent)24. Since allopatric speciation is the outcome of dispersal limitation, the effect of dispersal on the relationship between phylogenetic turnover and spatial/barrier distance appears to reverse in direction between regional and continental scales. However, the effect of allopatric speciation on phylogenetic turnover should also be relatively ephemeral48; as soon as sister lineages on each side of the barrier start to diversify into clades, phylogenetic turnover becomes positively related to barrier distance. Cases of relatively recent allopatric speciation are documented in American palms31 but clearly do not dominate our results. This might partly be due to the lack of terminal (intrageneric) phylogenetic resolution and/or our choice of phylogenetic turnover metric, which is known to emphasize ‘basal’ phylogenetic relationships49. However, given the large extent of the study and the long presence of palms in the Americas, it is likely that any signal of allopatric speciation would be weak compared to the diversification of clades within regions that are delineated by barriers18. Still, there might be some effect of allopatric speciation that decreases the correlation between phylogenetic turnover and spatial/barrier distance. A similar logic applies to adaptive speciation: if sister species segregate along spatially structured environmental gradients50 phylogenetic turnover might actually decrease with environmental dissimilarity (M2 in Table 1). Again, this is most likely to occur at spatial scales much smaller than the scale of our study. In any case, our interpretations are conservative: if allopatric or adaptive speciation influenced phylogenetic turnover in our study system, it would bias the slopes and fractions of variation presented here towards zero and insignificance.
Our findings allow some thoughts on the future of American palm diversity under climate change. If niches evolve slowly even on evolutionary timescales species will not readily adapt to rapidly changing environments. Climate-related genetic variation within species appears to be widespread51 and rapid adaptation to climate change occurs52. Adaptation at the population level through selection acting on existing genotypes may be rapid, but adaptation to truly new environments is limited by the supply of new genotypes through mutation51. The former is conceivable even for groups showing strong phylogenetic niche conservatism, but only until the climatic changes extend beyond the range of conditions that is covered by existing intra-specific niche variation. At the end of the day, adaptation to environments that are not already part of the species' niche may be the key to surviving dramatic, rapid climate change. Our data suggested that this type of adaptation might be limited in American palms; however, more studies are required to improve our understanding of the adaptive capacities within this charismatic, ecologically and economically important plant group towards a changing world.
In conclusion, we found that the turnover of clades within American palms is controlled by a combination of two factors: 1) tolerances to temperature extremes and seasonality and soil preferences, evolve slowly compared to the tempo of lineage diversification and 2) dispersal is limited on evolutionary timescales, definitely by barriers and possibly by time. The results corroborate that phylogenetic history is pivotal for understanding large-scale diversity patterns, especially since the turnover of clades can have important downstream effects on patterns of species richness and functional diversity. Future studies on other groups of organisms should also take into account that diversity patterns may be strongly shaped by restricted lineage mobility during diversification. Of note, the balance of dispersal limitation and phylogenetic niche conservatism, as well as the relevant niche factors, might be very different in other taxa due to ecological and life history differences and in other regions due to differences in geomorphology, environment and history. We found that ‘barrier distance’, our measure of dispersal barriers between locations, is a more relevant predictor of phylogenetic turnover than spatial distance. This measure should be similarly useful for studies on species turnover. Finally, because their dispersal and niche evolution are limited on evolutionary timescales, palms might fail to migrate or adapt in response to rapidly changing climates. This would have serious consequences for tropical biodiversity and for cultures that depend on palms as a resource53,54.
Methods
An overview of the analyses performed for this article is shown in Fig. 4.
Workflow of the analyses carried out for this study.
Each individual step is described in the text (Methods section). Re: variation in phylogenetic turnover explained by environmental dissimilarity, Rd: explained by spatial/barrier distance, Rt: explained by environmental dissimilarity and spatial/barrier distance. Rpe: explained by environmental dissimilarity alone, Rpd: explained by spatial/barrier distance alone, Rmx: explained by either environmental dissimilarity or spatial/barrier distance, Run: unexplained. PCA = Principal Components Analysis.
Distribution data
We used distribution maps of all American palms18, excluding three that are endemic to remote islands with no other palms (Brahea edulis, Juania australis, Sabal bermudana). This resulted in a total of 547 species. Due to taxonomic developments, close to 800 species of palms are currently recognized for the Americas17. However, there are no comparable distribution data that match the more recent taxonomy and we are not aware of any bias in taxonomic changes since 1995 that might influence our results. We registered presence or absence of the included 547 species within 1° × 1° grid cells (n = 1701 cells with palms, Fig. 1a). We preferred this grid to an equal area grid because it has previously been used in analyses of palm species richness14,55, turnover56 and net diversification57. Thus, we accepted small area differences among grid cells for the sake of comparability. The richness per grid cell ranged from 1 to 83 species (Fig. 1a)14. These cells were used as assemblages in the analysis of phylogenetic turnover.
Phylogenetic tree
We modified a recently published dated version58 of a genus level phylogenetic tree of all palms59 to fit our assemblage dataset. We deleted genera that did not occur in the Americas and replaced terminal branches that represented genera with > 1 species with polytomies that included all species in our dataset. The crown age of those polytomies had to be chosen arbitrarily; to test the influence of this parameter on our results we produced four versions: crown ages equalling 10%, 50% and 90% of the genus stem age and crown ages computed with a simulation approach60. The variation caused by to those different assumptions was orders of magnitude smaller than the values of interest and did not influence the qualitative outcome of our study. Thus, we present only the results for genus crown ages set to 50% of the stem age. Other related measures of phylogenetic community structure have been found equally insensitive to this parameter20.
Phylogenetic turnover
For all pairwise combinations of grid cells, the comdist metric48 was calculated in R (http://www.r-project.org/). We modified the comdist function of the package picante61 to improve computational performance (see Supplementary Methods online). Comdist is defined as:

where piA is the presence ( = 1) or absence ( = 0) of species i in assemblage A; dij is the phylogenetic distance between species i and j; A is the species richness of assemblage A. In our case, dij was proportional to pairwise divergence time; therefore, comdist represented the time in the past when the members of assemblage A diverged, on average, from the members of assemblage B.
Environmental variables
We extracted environmental information for each grid cell from Geographic Information System (GIS) layers with ArcInfo 9.3 (ESRI Inc., Redlands, California, USA). We obtained a total of 19 climatic variables, 6 soil variables, 2 topographic variables and 4 measures of vegetation structure (see Supplementary Table S4 for information on the sources). All variables were averaged for each grid cell, except elevation, where a range was calculated to reflect topographic heterogeneity. All variables were standardised and centred.
Environmental dissimilarity
First, we computed a measure of environmental dissimilarity where all variables were included on an equal basis. We subjected the dataset to a principal components analysis (PCA) to remove multicollinearity between variables; all components with standard deviations < 10% of the standard deviation of the first component were omitted. Environmental dissimilarity was then calculated as the Euclidean distance in the space spanned by the PCA axes. Second, we repeated this procedure for five separate sets of variables, including temperature (bioclimatic variables 1–11), precipitation (bioclimatic variables 12–19), soil, topography and vegetation structure (QSCAT and NDVI). Third, we computed a separate dissimilarity matrix for each environmental factor, with Euclidean distances taken directly from the standardised variables. This resulted in a total of 37 matrices of environmental dissimilarity for all pairwise combinations of grid cells.
Dispersal limitation
We computed two different measures to represent the degree to which dispersal among grid cells was limited. First, we calculated geodesic distance between grid cell midpoints of all pair-wise combinations (‘spatial distance’). Second, we calculated a ‘barrier distance’ that takes the environmental insuitability of interjacent areas (i.e. dispersal barriers sensu ref. 27) into account. For each grid cell, a GIS layer was calculated for the entire study area representing how different the environment is from the environment of the focal grid cell (‘cost surface’). For each pair of grid cells, environment-based dispersal distance was calculated as the average length of the least-cost path between them, based on their respective cost surfaces. Details on the calculation can be found in the Supplementary Methods online.
Analyses
We used ordinary least squares linear regression models to determine whether environmental dissimilarity (de) and spatial/barrier distance (dd) could explain the variation in phylogenetic turnover (dφ). As dd we used either spatial distance or barrier distance. Three models were built, with dφ ~ de, dφ ~ dd and dφ ~ de + dd. All variables were centred and standardised to obtain comparable effect sizes. We used variation partitioning62 to divide the variance of phylogenetic turnover into four fractions, according to whether they could be explained by environmental dissimilarity alone (Rpe), spatial/barrier distance alone (Rpd), environmental dissimilarity and dispersal distance (Rmx), or unexplained (Run). These fractions were computed from the R2 values of the dφ ~ de model (Re), the dφ ~ dd model (Rd) and the multiple predictor model (Rt) as follows: Rpe = Rt − Rd; Rpd = Rt − Re; Rmx = Re + Rd − Rt; and Run = 1 − Rt.
Significance testing
Since pairwise measures of dissimilarity are not statistically independent, we used a permutation procedure to assess significances. This allowed us to account simultaneously for a second problem, namely that phylogenetic turnover between assemblages is confounded by the number of shared species (i.e. species turnover) as well as differences in species richness. For the purpose of our study, we were only interested in the turnover of higher-level lineages, not species. We therefore employed a null model where we re-shuffled the species labels on the phylogeny 999 times and re-calculated phylogenetic turnover from these randomised phylogenies. For each of these null matrices, we performed the regression analysis and variation partitioning described above. In these null analyses, comdist still correlated with environmental dissimilarity and dispersal distance due to shared species and richness differences. The proportion of times that the Rpe and Rpd fractions, respectively, of the null analyses were higher than the Rpe/Rpd fractions of the real data was used as the probability of the hypothesis H0 that niche evolution/dispersal limitation did not affect phylogenetic turnover. This procedure has been previously used to test for relationships between phylogenetic turnover and altitudinal difference independent of species turnover23.
References
Lomolino, M. V., Riddle, B. R., Whittaker, R. J. & Brown, J. H. Biogeography. 4 edn, (Sinauer Associates, 2010).
Ackerly, D. D., Schwilk, D. W. & Webb, C. O. Niche evolution and adaptive radiation: Testing the order of trait divergence. Ecology 87, S50–S61 (2006).
Pennington, R. T., Lavin, M. & Oliveira, A. Woody plant diversity, evolution and ecology in the tropics: Perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Evol. S. 40, 437–457 (2009).
Buckley, L. B. et al. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proc. Roy. Soc. B-Biol. Sci. 277, 2131–2138 (2010).
Roncal, J., Blach-Overgaard, A., Borchsenius, F., Balslev, H. & Svenning, J. C. A dated phylogeny complements macroecological analysis to explain the diversity patterns in Geonoma (Arecaceae). Biotropica 43, 324–334 (2011).
Stephens, P. R. & Wiens, J. J. Bridging the gap between community ecology and historical biogeography: niche conservatism and community structure in emydid turtles. Mol. Ecol. 18, 4664–4679 (2009).
Wiens, J. J. The causes of species richness patterns across space, time and clades and the role of "ecological limits". Q. Rev. Biol. 86, 75–96 (2011).
Baselga, A., Lobo, J. M., Svenning, J.-C., Aragón, P. & Araújo, M. B. Dispersal ability modulates the strength of the latitudinal richness gradient in European beetles. Global Ecol. Biogeogr. 21, 1106–1113 (2012).
Wiens, J. J. & Donoghue, M. J. Historical biogeography, ecology and species richness. Trends. Ecol. Evol. 19, 639–644 (2004).
Antonelli, A. & Sanmartin, I. Why are there so many plant species in the Neotropics? Taxon 60, 403–414 (2011).
Hoorn, C. et al. Amazonia Through Time: Andean Uplift, Climate Change, Landscape Evolution and Biodiversity. Science 330, 927–931 (2010).
Pennington, R. T., Richardson, J. E. & Lavin, M. Insights into the historical construction of species-rich biomes from dated plant phylogenies, neutral ecological theory and phylogenetic community structure. New Phytol. 172, 605–616 (2006).
Rull, V. Neotropical biodiversity: timing and potential drivers. Trends Ecol. Evol. 26, 508–513 (2011).
Bjorholm, S., Svenning, J. C., Baker, W. J., Skov, F. & Balslev, H. Historical legacies in the geographical diversity patterns of New World palm (Arecaceae) subfamilies. Bot. J. Linn. Soc. 151, 113–125 (2006).
Webb, S. D. The Great American Biotic Interchange: Patterns and processes. Ann. Mo. Bot. Gard. 93, 245–257 (2006).
Kissling, W. D. et al. Quaternary and pre-Quaternary historical legacies in the global distribution of a major tropical plant lineage. Global Ecol. Biogeogr. 21, 909–921 (2012).
Balslev, H. et al. Species diversity and growth forms in tropical American palm communities. Bot. Rev. 77, 381–425 (2011).
Henderson, A., Galeano, G. & Bernal, R. Field guide to the palms of the Americas. (Princeton University Press, 1995).
Eiserhardt, W. L., Svenning, J.-C., Kissling, D. & Balslev, H. Geographical ecology of the palms (Arecaceae): determinants of diversity and distributions across spatial scales. Ann. Bot. 108, 1391–1416 (2011).
Kissling, W. D. et al. Cenozoic imprints on the phylogenetic structure of palm species assemblages worldwide. P. Natl. Acad. Sci. USA 109, 7379–7384 (2012).
Graham, C. H. & Fine, P. V. A. Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecol. Lett. 11, 1265–1277 (2008).
Tuomisto, H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33, 2–22 (2010).
Bryant, J. A. et al. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. P. Natl. Acad. Sci. USA 105, 11505–11511 (2008).
Graham, C. H., Parra, J. L., Rahbek, C. & McGuire, J. A. Phylogenetic structure in tropical hummingbird communities. P. Natl. Acad. Sci. USA 106, 19673–19678 (2009).
Parmentier, I. & Hardy, O. J. The impact of ecological differentiation and dispersal limitation on species turnover and phylogenetic structure of inselberg's plant communities. Ecography 32, 613–622 (2009).
Baker, W. J. & Couvreur, T. L. P. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. II. diversification history and origin of regional assemblages. J. Biogeogr. 40, 286–298 (2013).
Wiens, J. J. & Graham, C. H. Niche conservatism: Integrating evolution, ecology and conservation biology. Annu. Rev. Ecol. Evol. S. 36, 519–539 (2005).
Nekola, J. C. & White, P. S. The distance decay of similarity in biogeography and ecology. J. Biogeogr. 26, 867–878 (1999).
Svenning, J.-C. & Skov, F. Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol. Lett. 10, 453–460 (2007).
Paul, J. R., Morton, C., Taylor, C. M. & Tonsor, S. J. Evolutionary time for dispersal limits the extent but not the occupancy of species' potential ranges in the tropical plant genus Psychotria (Rubiaceae). Am. Nat. 173, 188–199 (2009).
Barfod, A. S., Trenel, P. & Borchsenius, F. in Diversity, Phylogeny and Evolution of the Monocotyledons. (eds O. Seberg, G. Petersen, A. S. Barfod, & J. I. Davis ) 225–243 (Aarhus University Press, 2010).
Cuenca, A., Asmussen-Lange, C. B. & Borchsenius, F. A dated phylogeny of the palm tribe Chamaedoreeae supports Eocene dispersal between Africa, North and South America. Mol. Phylogenet. Evol. 46, 760–775 (2008).
Roncal, J., Borchsenius, F., Asmussen-Lange, C. B. & Balslev, H. in Diversity, phylogeny and evolution of the monocotyledons. (eds O. Seberg, G. Petersen, A. S. Barfod, & J. I. Davis) 245–265 (Aarhus University Press, 2010).
Losos, J. B. Seeing the forest for the trees: The limitations of phylogenies in comparative biology. Am. Nat. 177, 709–727 (2011).
Wiens, J. J. et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (2010).
Peterson, A. T., Soberon, J. & Sanchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 (1999).
Kellermann, V. et al. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. P. Natl. Acad. Sci. USA, early online, doi: 10.1073/pnas.1207553109.
Kozak, K. H. & Wiens, J. J. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am. Nat. 176, 40–54 (2010).
Woodward, F. I., Fogg, G. E. & Heber, U. The impact of low temperatures in controlling the geographical distribution of plants [and discussion]. Philos. T. R. Soc. B 326, 585–593 (1990).
Walther, G. R. et al. Palms tracking climate change. Global Ecol. Biogeogr. 16, 801–809 (2007).
Salm, R., Salles, N. V. D., Alonso, W. J. & Schuck-Paim, C. Cross-scale determinants of palm species distribution. Acta Amazonica 37, 17–25 (2007).
Tomlinson, P. B. The uniqueness of palms. Bot. J. Linn. Soc. 151, 5–14 (2006).
Blach-Overgaard, A., Svenning, J. C., Dransfield, J., Greve, M. & Balslev, H. Determinants of palm species distributions across Africa: the relative roles of climate, non-climatic environmental factors and spatial constraints. Ecography 33, 380–391 (2010).
Prinzing, A., Durka, W., Klotz, S. & Brandl, R. The niche of higher plants: evidence for phylogenetic conservatism. Proc. Roy. Soc. B-Biol. Sci. 268, 2383–2389 (2001).
Buermann, W. et al. Predicting species distributions across the Amazonian and Andean regions using remote sensing data. J. Biogeogr 35, 1160–1176 (2008).
Tuomisto, H. & Ruokolainen, K. Analyzing or explaining beta diversity? Reply. Ecology 89, 3244–3256 (2008).
Hardy, O. J. & Senterre, B. Characterizing the phylogenetic structure of communities by an additive partitioning of phylogenetic diversity. J. Ecol. 95, 493–506 (2007).
Webb, C. O., Cannon, C. H. & Davies, R. G. in Tropical forest community ecology. (eds W. P. Carson & S. A. Schnitzer) 79–97 (Wiley & Sons, 2008).
Swenson, N. G. Phylogenetic beta diversity metrics, trait evolution and inferring the functional beta diversity of communities. PLoS ONE 6, e21264 (2011).
Fine, P. V. A., Daly, D. C., Munoz, G. V., Mesones, I. & Cameron, K. M. The contribution of edaphic heterogeneity to the evolution and diversity of Burseraceae trees in the western Amazon. Evolution 59, 1464–1478 (2005).
Jump, A. S. & Peñuelas, J. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (2005).
Franks, S. J., Sim, S. & Weis, A. E. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. P. Natl. Acad. Sci. USA 104, 1278–1282 (2007).
Balick, M. J. & Beck, H. T. Useful palms of the world: a synoptic bibliography (Columbia University Press, New York, 1990).
Balslev, H. Palm harvest impacts in north-western South America. Bot. Rev 77, 370–380 (2011).
Eiserhardt, W. L., Bjorholm, S., Svenning, J. C., Rangel, T. F. & Balslev, H. Testing the water-energy theory on American palms (Arecaceae) using geographically weighted regression. PLoS ONE 6, e27027 (2011).
Bjorholm, S., Svenning, J.-C., Skov, F. & Balslev, H. To what extent does Tobler's 1st law of geography apply to macroecology? A case study using American palms (Arecaceae). BMC Ecology 8, 11 (2008).
Svenning, J. C., Borchsenius, F., Bjorholm, S. & Balslev, H. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr. 35, 394–406 (2008).
Couvreur, T. L. P., Forest, F. & Baker, W. J. Origins and global diversification patterns of tropical rain forests: inferences from a complete genus-level phylogeny of palms. BMC Biol. 9, 44 (2011).
Baker, W. J. et al. Complete generic-level phylogenetic analyses of palms (Arecaceae) with comparisons of supertree and supermatrix approaches. Syst. Biol. 58, 240–256 (2009).
Eiserhardt, W. L., Svenning, J.-C., Borchsenius, F., Kristiansen, T. & Balslev, H. Separating environmental and geographical determinants of phylogenetic community structure in Amazonian palms (Arecaceae). Bot. J. Linn. Soc. 171, 244–259 (2013).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010).
Legendre, P. & Legendre, P. Numerical Ecology. 2nd. Edition edn (Elsevier, 1998).
Acknowledgements
This work was supported by the Danish Council for Independent Research - Natural Sciences [grant# 272-07-0242 to JCS, #271-06-0476 to HB]; the European Community [FP7 grant #212631 to HB]; the Villum Kahn Rasmussen Foundation [to JCS]; and The Aarhus University Research Foundation (AUFF) [to WLE]. All maps were drawn using Arc GIS and annotated in Incscape.
Author information
Authors and Affiliations
Contributions
W.L.E., J.-C.S. and H.B. developed the idea. W.J.B. and T.L.P.C. constructed the dated phylogeny. W.L.E. performed the analyses and led the writing. All authors contributed substantially to revisions.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Eiserhardt, W., Svenning, JC., Baker, W. et al. Dispersal and niche evolution jointly shape the geographic turnover of phylogenetic clades across continents. Sci Rep 3, 1164 (2013). https://doi.org/10.1038/srep01164
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01164
This article is cited by
-
Anthropogenic threats and habitat degradation challenge the conservation of palm genetic resources—an appraisal of current status, threats and look-ahead strategies
Biodiversity and Conservation (2023)
-
The contribution of environmental and dispersal filters on phylogenetic and taxonomic beta diversity patterns in Amazonian tree communities
Oecologia (2021)
-
Rapid climate change results in long-lasting spatial homogenization of phylogenetic diversity
Nature Communications (2020)
-
Environment and evolutionary history shape phylogenetic turnover in European tetrapods
Nature Communications (2019)
-
Environmental filtering of eudicot lineages underlies phylogenetic clustering in tropical South American flooded forests
Oecologia (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.