Abstract
Gas-bubble lesions were described in cetaceans stranded in spatio-temporal concordance with naval exercises using high-powered sonars. A behaviourally induced decompression sickness-like disease was proposed as a plausible causal mechanism, although these findings remain scientifically controversial. Investigations into the constituents of the gas bubbles in suspected gas embolism cases are highly desirable. We have found that vacuum tubes, insulin syringes and an aspirometer are reliable tools for in situ gas sampling, storage and transportation without appreciable loss of gas and without compromising the accuracy of the analysis. Gas analysis is conducted by gas chromatography in the laboratory. This methodology was successfully applied to a mass stranding of sperm whales, to a beaked whale stranded in spatial and temporal association with military exercises and to a cetacean chronic gas embolism case. Results from the freshest animals confirmed that bubbles were relatively free of gases associated with putrefaction and consisted predominantly of nitrogen.
Similar content being viewed by others
Introduction
Gas-bubble lesions have been described in cetaceans stranded in spatio-temporal concordance with military maneuvers1,2. Authors described an acute and systemic gas and fat embolic syndrome similar to decompression sickness (DCS) in human divers. These findings raised a number of questions addressing cetacean's ability to avoid hyperbaric conditions like DCS. Two key questions arising from such findings are: a) What are the constituent gases in the bubbles?3; (b) Do we have a robust method for gas analysis in stranded marine mammals?
Gas chromatography has been demonstrated as a valid method to discriminate putrefaction gases from air embolism4,5 and has been used as a forensic tool in humans for this purpose6. Attempts have been made to analyze the gas produced during decompression, using a wide variety of methods in humans and experimental animal pathology but not, so far, in cetaceans7,8,9,10,11,12,13. However, appropriate and accurate measurement of nitrogen and respiratory gases (any gas that has an active role in the respiratory process such as oxygen or CO2) while avoiding atmospheric air is difficult. Additionally, cetaceans might strand on beaches that are not easily accessible and might require that necropsy is performed in situ.
To our knowledge, no transportable apparatus exists that can simultaneously measure in an accurate manner respiratory gases as well as hydrocarbons produced by the metabolism of microorganisms involved in post mortem putrefaction phenomena. Therefore, although gas extraction can sometimes be performed at the stranding site, gas analysis must always take place in a laboratory, a situation that requires proper storage and transportation of gas samples. Gases likely to be of scientific interest might be found in sites within body cavities (such as intestinal gas, air in sinuses, pneumothorax, subcapsular gas or gas pockets), inside vessels (emboli) and/or mixed with blood inside the heart. Sampling of gas from these body sites will require the use of different techniques.
In the present paper, we tested different materials and tools through appropriate laboratory validations, in order to develop a standardized methodology for in situ extraction and transportation, as well as for the subsequent laboratory analysis of the gas constituents in gas embolism cases via a single injection of stored gas into a gas chromatograph. Glass containers, plastic insulin syringes and a new aspirometer design filled with different liquid barriers was tested for reliability in gas sample collection and storage. Vacutainers®, commercially available vacuum-containing tubes of non-breakable glass, are good for sampling gas from sites within body cavities. They were studied for their suitability for the conservation of gas samples from marine mammal carcasses due to their industrial vacuum and gas-tight seal. However, vacutainers® do not contain a complete vacuum, thus traces of background air are needed to be quantified and corrected for. Syringes are needed to sample the gas bubbles found in veins. With vacuum tubes, blood will enter the tube together with the gas bubble. Further autolysis and putrefaction of the blood will contaminate the gas sample. On the other hand, syringes enable us to sample different gas volumes in a control manner and to measure it. Glass tight syringes are a potential liability for fieldwork conditions and so disposable insulin syringes were also tested for practicality of gas collection from blood vessels. However, sampling of gas from inside the heart with a syringe is difficult because of the opacity of the heart wall compare to veins. In this case, an aspirometer is very helpful.
The aspirometer, first described by Dyrenfurth14 to separate free gas from blood, has undergone several modifications by others but still needs to be improved for cetacean field work. For the new aspirometer design, we first reproduced the aspirometer described by Bajanowski et al. (1998), subsequently modifying it for gas collection during necropsy in the field. Bajanowski design's consists of an aspirator bottle connected to two joined flasks by a rubber tube. The joined flasks consist on an aspirator bottle in the base joined to a gas burette in the top. This aspirator bottle has an additional lateral tube with a free end where the needle for puncture is placed. The burette has a glass stopcock in the upper part and is scaled for the measurement of the gas volume beginning from the most upper part where the gas will be collected. The stopcock is directly connected to a needle. This combination of aspirator bottle joint to a gas burette is what we have modified to make it more comfortable for fieldwork. The complete system must be filled with a barrier liquid, including the needle connected to the burette's stopcock and air bubbles must be removed. The barrier liquid is a liquid solution used to separate the free gas from the blood found in the heart cavities and to avoid atmospheric air pollution of the sample. In this study, the best barrier liquid through which to collect gas from the heart cavities during necropsy was also tested. The aspirometer needs to be made of a transparent material with no or a very limited number of surface cracks to avoid bubble attachment, this making the use of glass unavoidable.
Although the new protocol could also be used in human forensic science, the methodology has been specifically developed for the transportation of gas samples from cetacean stranding sites that are not readily accessible to humans. Once developed, this methodology was utilized for a mass stranding of sperm whales (Physeter macrocephalus) along the coast of Italy, as well as for a single stranding of a Cuvier's beaked whale (Ziphius cavirostris) along the Spanish coastline in temporal and spatial association with military (naval) exercises and for a Risso's dolphin (Grampus griseus) found stranded on the United Kingdom (UK) coastline and affected by chronic gas embolic lesions.
Results
Testing of gas storage methods
The gas content of 5-mL additive-free vacutainers® was analysed and compared to the gas contents of manually helium-purged 2-mL containers. The background air content of the vacuum tubes (measured as the area resulting from the integration of the chromatographic detector signal within a specific retention time, area counts) was found to be only slightly lower (2033 ± 25) than the helium containers (2171 ± 587 area counts), although its standard deviation (SD) was much smaller.
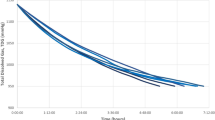
Secondly, the background and diffusion rate of air (area counts) in different vacuum tubes (3.5 with additives vacutainer® vs 5-mL additive-free vacutainers®) was compared over time up to 21 days at room temperature (Fig. 1). In the resultant graph, we considered the following log-linear model (equation (1)), with an adjustment of R2 = 0.944. All estimated parameters had a P-value less than 0.001.


where type = 1 or 0 according to whether the vacutainer® type is with or without air. No interaction was found for the proposed model (P = 0.153). Therefore, the diffusion rate was the same in both vacutainer® types and calculated from the model as  .
.

Loss of gas sample within vacuum tubes occurred at a rate of 0.99% (95% CI = 0.989%, 0.992%) per day. Differences among vacutainers® were found in the amount of background air, with the 5 mL additive-free vacutainer® exhibiting the lowest background air volume.
Thirdly, the best storage temperature was determined by the comparison of air amount (area counts) over time in 60 vacutainers® stored at different temperatures: room temperature (20–24°C), refrigerator (4°C) and freezer (−80°C). An interaction between temperature and day was found on day 14 among all investigated samples. Apart from day 14, the mean values for oxygen and nitrogen content were found to be similar within the same temperature throughout time. No statistically significant differences were found. Conversely, differences were observed among temperatures, with room and refrigerator mean values being similar, while being clearly different from freezer values (Table 1).
In order to observe whether puncturing through the rubber could affect in a later stage gas conservation inside the vacutainer®, 24 vacutainers® were sampled on two separate occasions. An increase in air content (area counts) was found at all temperatures, with the freezer samples being the most increased and the room temperature samples being the least increased, respectively.
In addition, potential differences in diffusion rate of different gases (oxygen, nitrogen, CO2 and H2) through the 5-mL additive-free vacutainer® were also evaluated at constant room temperature (21°C) for 5 days. Gas content (area counts) was compared to day one. No differences were found since mean values for each day were always within the range of 0.95 to 1.05 (Fig. 2a–2c). Dispersion of the data was found to be inversely correlated to the detector sensibility, with hydrogen (the gas with poorest detection) being the one with more disperse data. However, variability of the data was much lower when calculating ratios (N2/O2) rather than absolute values. As a result, the gas composition was very stable (Fig. 2d). To simulate a sample composed of a mixture of all these gases, the average values of each individual gas constituent for a given day were summed and the average values were used to calculate changes in the relative percentage (relative %) of each gas in the mixture over the same time period. There was no significant variation in relative % of oxygen, nitrogen, CO2 and H2 over the 5 days period.
Finally, vacutainers® were tested for their suitability for transportation in aircraft. No significant differences were found for either oxygen (P = 0.137) or nitrogen (P = 0.056) in control samples in vacutainers compared to samples of the same gases in vacutainers that were transported in the passenger cabin of a commercial jet aircraft.
Insulin syringe testing for gas sampling
In order to collect bubbles, disposable plastic insulin syringes were tested. The accuracy of these syringes for gas volume measurements was studied by promptly injecting different atmospheric air volumes (from 0 to 5 mL) into vacutainers® and analyzed by gas chromatography. Injected air volume (mL) was plotted against signals peak area (area counts) and a good linear regression was obtained (R2 = 0.9959).
The atmospheric air background of the insulin syringe and the possibility of atmospheric air pollution were also analyzed. Syringes were used for the extraction of 1 mL of pure helium, which was then injected into the vacutainers®. Air content was analyzed and compared to that of intact vacutainers®. No significant differences were found for either oxygen (P = 0.336) or nitrogen (P = 0.337) content (area counts).
New aspirometer design
The aspirometer described by Bajanowski et al. (1998) was basically modified for practicality in the field. Instead of having an aspirator bottle joined to a gas burette, we designed a reverse gas burette with a hexagonal or rounded base, similar to those of graduated cylinders for easier handling and better support. This simple modification eliminates one possible mode of atmospheric air entrance and at the same time reduces slopes of surface contact where small bubbles sometimes become attached, thus maximizing gas sample size recovery. This new design (U201100896) maximizes sample size recovery and makes handling easier, while the hexagonal or rounded base gives stability to the tool (Fig. 3). Changes in temperature and pressure can throttle the glass stopcock, wherefore we have change the material used for the stopcock from gas to Teflon.
Testing liquid barriers for gas in blood
The aspirometer must be completely filled with a gas liquid barrier to separate the free gas from the blood found in the heart cavities and to avoid atmospheric air pollution of the sample. Distilled water and an aqueous solution of 20%wt NaCl with a pH of 4 were tested as barrier liquids. Differences were found, showing an increase in oxygen and nitrogen and a marked decrease in CO2, when the gas was forced to cross through the distilled water of the aspirometer, while hydrogen was fully recovered. These differences were greatly reduced when using the aqueous 20%wt NaCl solution (Table 2).
Stranded cetaceans necropsied and sampled for gas
A standard necropsy protocol was used, with some modifications necessary to preserve bubbles for gas collection and storage. Skin and blubber were removed taking into account possible gas embolism within the subcutaneous veins. If bubbles were seen, vessels were explored to confirm that they had not been cut during dissection. As soon as a bubble was detected, a photograph was taken and the bubble was sampled for gas analysis. In order to have access to the body cavities, dissection was done avoiding cutting of medium-large vessels (usually larger than 3 mm, although it varies among species). The abdominal cavity was opened first and mesenteric and renal veins as well as the lumbo-caudal venous plexus were screened for bubbles. The thoracic cavity was then opened to permit access to the heart and coronary veins were explored. After bubble exploration and gas sampling from the different localizations, systemic vessels could be subsequently cut and the routine necropsy protocol could be completed.
When sampling gas from sites within body cavities, the 5-mL additive-free vacutainer (BD Vacutainer® Z. ref: 367624) was directly applied to cavities with its appropriate plastic holder or adapter and a double-pointed needle with a rubber barrier on the tube puncture side (ref: 360214). To avoid atmospheric air, the needle was preliminarily inserted into the cavity for purging; the vacutainer® was then pushed against the double-pointed needle and, finally, the vacutainer® was removed before the needle was released from the cavity. This technique allowed adequate gas sampling from head sinuses, the digestive tract and even from heart chambers if post mortem autolysis ranged from grade three to grade five15. The pericardial sac was always filled with distilled water to avoid contamination with atmospheric air.
If the carcass preservation status was fresh or very fresh (putrefaction grades 2 or 1, respectively), then the aspirometer was used to separate the gas from the blood found in the heart. In order to properly sample bubbles in the rest of the cardiovascular system (blood vessels), disposable insulin syringes (BD Plastipak U-100 insulin) were used to sample bubbles and their contents were promptly injected into a vacutainer®. One new syringe and one new vacutainer® were used for each bubble.
In the mass stranding of sperm whales (Physeter macrocephalus) that occurred in December 2009 along the Southern Adriatic Sea coast of Italy3, a complete necropsy could be performed on the 3 out of the 7 sperm whales (SWs) that stranded alive for up to 48 hours. Gas bubbles were observed almost exclusively within the coronary heart veins. Pneumothorax and pneumomediastinum were also found, being more pronounced in the SWs that survived from prolonged lateral recumbence (up to 48 hours) before dying15 (Fig. 4). The gas composition of bubbles from the best-preserved sperm whale (SW 7) was 69.7% (±4.6%) N2, 15.3% (±6.8%) CO2 and 15.0% (±2.2%) O2 (n = 7), with SW 6 showing a gas composition of 61.5% (±6.9%) N2, 27.7% (±12.8%) CO2 and 10.7% (±6.0%) O2 (n = 5), while the most decomposed sperm whale (SW 5) exhibited a gas composition of 30.4% (±5.2%) N2 30.2% (±3.6%) CO2, 6.4% (±1.5%) O2 and 32.9% (±3.2%) H2 (n = 6). As the decomposition code increased, nitrogen and oxygen contents decreased, whereas CO2 content increased. After decomposition code 3, hydrogen also appeared. The gas emboli composition results were clearly different from intestinal gas composition for each animal. Intestinal gases were very variable in concentrations but nitrogen was absence or present in low quantities (<19 %). In SW 5 subcapsular intestinal emphysema was present. It was composed of 58.0% (±0.5%) H2, 26.5% (±3.8%) CO2 and 2.9% (±2.8%) O2 (n = 2) (Fig. 5).
External view of one of the stranded sperm whales (a), one of which showing pneumodiastinum (b); external view of the Cuvier's beaked whale (c), close-up view of intravascular bubbles in the coronary heart veins of the beaked whale (d), external view of the Risso's dolphin (e), abdominal cavity with close-up view of the spleen showing chronic gas embolism (f).
Composition of gas samples taken from sperm whales that mass stranded in Italy (SW 7, SW 6 and SW 5), as well as to a single animal stranded on the coast of the Canary Islands (CET 520).
Samples were taken from the coronary heart veins and from the intestinal lumen in the 4 whales. Subcapsular intestinal emhysema was additionally sampled in SW 5. Gas composition is expressed as the percentage of the average mole fraction. Gas samples are order in the ordinate axis first by localization of the sample and later by decomposition codes.
Gas analysis was additionally performed in a Cuvier's beaked whale (Ziphius cavirostris) that stranded alive in shallow waters in Spain on March of 2011 in temporal and spatial association with military exercises using high-intensity, anti-submarine sonar. Necropsy was conducted within 24 hours of death and demonstrated severe and systemic acute gas and fat embolic lesions consistent with DCS (Fernández et al., submitted) (Fig. 4). Mean gas proportions for bubbles in the coronary veins (n = 3), renal veins (n = 3), right ventricle (n = 2) and right atrium (n = 1) contained predominantly N2 (n = 9; mean = 73.3 ± 5.7 %), with a very low proportion of putrefaction gases such as H2 (n = 9; mean = 4.7±4.9%). Relative proportions were determined for O2 (n = 9; mean = 9.7±2.6%) and CO2 (n = 9; mean = 12.2±6.3%). Gas bubbles from the mesenteric veins (n = 4) were more heterogeneous, with a higher percentage of putrefaction gases. These bubbles were composed by 37.8–65.3% nitrogen, 10.8–17.6% H2, 5.2–12.9% O2 and 10.7–39.5% CO2.
Finally, gas samples (n = 9) were taken from the massively enlarged and gas cavitated spleen of a Risso's dolphin (Grampus griseus), found stranded on the UK coast in September 2009. The whole spleen from this animal was stored frozen (−20°C) prior to gas sample collection and analysis. The spleen showed severe chronic gas embolic lesions that were mainly composed of nitrogen (94.4±1.7%) (Fig. 4). Other gases found were oxygen (4.7±1.7%) and CO2 (0.9±0.5%). Methane was detected only in trace levels (Fernández et al., submitted). The gas was enclosed by fibrous tissue enabling careful dissection of the organ under distilled water to collect the gas samples.
Discussion
This is the first time that a methodology for gas storage and analysis has been developed and experimentally verified for post mortem investigation of marine mammals. This study was able to demonstrate the optimum conditions for gas sampling and storage in cheap and commercially available vacutainers® without contamination of atmospheric air – even when samples are transported on a commercial aircraft. This study was also able to determine the optimum conditions for sampling gas from different body compartments including tissues and blood. A new designed glass aspirometer (U201100896) is needed to collect gas from the heart across a fluid barrier and plastic insulin syringes were also shown to be viable for collecting bubbles from blood vessels, provided the gas sample is rapidly injected into plain vacutainers® for storage prior to analysis of gas contents via gas chromatography.
On the basis of the results obtained from stranded cetaceans and presented herein, differences in gas composition were noted between fresh tissue samples, putrefied tissue samples and intestinal gases, thus demonstrating that in situ gas sampling and storage may be possible provided that adequate materials (e.g. glass for storage) are employed, as well as by calculating the possible interferences that may occur with the gas sample all along the process and by correcting them into the calculations. One of these interferences with the gas sample was found in the vacutainers®: they contained residual air. The presence of oxygen and nitrogen in the vacuum tubes, which has been previously described, represents a problem for blood gas determinations16,17. Since oxygen and nitrogen levels are very important from the diagnostic point of view for gas embolism4,6, it is of crucial importance to calculate the air background levels of the tubes and to correct for it in subsequent calculations. For this purpose, we carried one blank vacuum tube (a vacutainer® into which nothing has been injected) along with each gas sample collected through the entire process. This means that both the sample and the blank container experienced the same changes in temperature and pressure. This also enables us to introduce a correction factor which is essentially a detection limit set at  , where
, where  is the minimum detectable signal,
is the minimum detectable signal,  is the average signal for a given gas in the blanks and
is the average signal for a given gas in the blanks and  is the associated standard deviation (SD)18. Since SD is very important, vacuum tubes are better candidates for gas storage than helium containers, due to the smaller SD of the air background.
is the associated standard deviation (SD)18. Since SD is very important, vacuum tubes are better candidates for gas storage than helium containers, due to the smaller SD of the air background.
By the same reasoning, lower background air contents in vacutainers® provide higher resolution for analyzing small samples. According to our model, the 5-mL additive-free vacutainer® has the smallest background and both vacutainer® types exhibit the same diffusion rate; therefore, we recommend using the 5-mL additive-free vacutainer®. As a guideline, we observed that bubbles larger than 0.5 mL usually give good signals when using this type of vacutainer®, although smaller volumes (0.35 mL approximately) have been analyzed successfully. Therefore we recommend to sample bubbles larger than 0.5 mL when using the method here described. It is important to note that, when measuring the blank vacutainers®, we are also correcting for the background air in the gas-tight syringe that is used for manual injection of gas into the gas chromatograph.
In relation to temperature storage, average values from day 21 were similar to those from day 1, so the interaction found on day 14 was considered incidental. Some fluctuation in the equipment may have occurred on or around that day. The lowest variability in data was found at room temperature conditions, both throughout time and when samples were exposed to several injections.
The diffusion rate was found to be similar for the different gases held in storage in vacutainers®, with no appreciable difference being observed when calculating relative proportions instead of total amounts. The diffusion rate calculated from our model at room temperature was equivalent to 0.9% per day. Molloy et al. (1973) reported a similar value (1%) for gas-tight bottles kept at room temperature19. This low diffusion rate, together with the non-appreciable difference for relative proportions, indicates that samples may be stored for several days without a relevant loss of gas and without affecting the accuracy of the analysis. However, in order to achieve more accurate results, it is highly recommended that the gas analysis be performed as soon as possible and that relative quantities be calculated instead of absolute values.
Results of gas samples transported by aircraft in vacutainers® indicate that there were no statistical differences and thus they can be transported by plane. Nevertheless, nitrogen P value (P = 0.056) was quasi-significant; therefore, it would be preferable to transport the samples inside some pressure resistance cage to ensure that no changes in gas composition will occur. The insulin syringe experiments demonstrated the accuracy of insulin syringes for gas volume measurements and showed that the syringe did not implement atmospheric air background on the methodology (with no statistically significant differences being detected). Knowles et al. (2006) studied the effects of syringe material, storage time and storage temperature on normal arterialized blood gas values20. One of the main conclusions of this work was that, for obtaining accurate and reliable results, samples drawn in plastic syringes should be analyzed immediately and that, if storage is required, samples should be kept in glass.
Although glass tight syringes are normally used under routine laboratory conditions, they are not suitable for fieldwork. Indeed, they are very fragile and easily blocked, so that it would be necessary to have one glass syringe per sample or to transport inert gas with which to purge the syringe between samplings and then store the sample elsewhere. By contrast, insulin syringes are made of plastic, making them disposable and very cheap. Handling of plastic material is always preferred versus glass during fieldwork. If a new syringe can be used for each bubble, purging is no longer necessary. Furthermore, since the use of plastic syringes appears to be fully compatible with storage in glass material (vacutainers®), in accordance with the findings of Knowles et al. (2006), we would strongly recommend the use of disposable syringes for fieldwork instead of gas tight syringes.
In the aspirometer, chemical interactions between the different barrier liquids tested and the gas sample were always found. Table 2 depicts a marked decrease in CO2 signal, along with an increase in nitrogen and oxygen, when the aspirometer is used. These differences may be explained by two simultaneous processes: solubilization of the gas compounds in the liquid barrier according to Henry's Law and dragging of the inherent gas (mainly nitrogen and oxygen) previously dissolved in distilled water.
Among the analyzed gases, only CO2 was noted to be significantly dissolved, with a scale change of 2.42. According to Henry's Law and applying a mass balance, its diffusion coefficient in distilled water at 24°C is 1.82. This value explains 75% of CO2 behavior. The remaining scale change might be explained by the chemical reaction of CO2, which is an acidic gas and reacts with distilled water as follows:

Alternatively, incorporation of the inherent liquid barrier gases could take place by dragging simultaneously to dilution. Therefore, instead of observing a slight decrease in nitrogen and oxygen signals according to Henry's Law, an increase was recorded. Nitrogen and oxygen are incorporated from the liquid barrier into the gas sample. Keil et al. (1980) also noted an incorporation of oxygen when storing gas samples in flasks with a liquid barrier21. Pulling of these gases could be due to molecular collisions between the gas sample and the inherent gas dissolved into solution. According to Tables 1 and 2, the chemical nature of the gas is not a factor since CO2 and hydrogen moved similar quantities of nitrogen and oxygen. Dyrenfurth (1928) proposed to boil distilled water just before its use14. Thermal and vacuum treatments were considered to diminish the amounts of nitrogen and oxygen diluted in the barrier liquid, but they were excluded. In this respect, it should be also noted it is not possible to transport the aspirometer filled with any liquid.
Additionally, correction factors may change depending on which solution is used, as well as in relation to water temperature and pH according to Henry's Law. In the Canary Islands, the ambient temperature is very constant during the year (20–24°C). These special climate circumstances allowed us to calculate a unique correction factor, but other researchers should consider the temperature range of the water in which they are working (Table 2). As shown in Table 2, recovery signals improved when an aqueous 20%wt NaCl solution was used; we strongly recommend this solution under normal circumstances, but not for cetaceans. Assays on the carcasses of deep diving cetaceans were unsuccessful when an aqueous 20%wt NaCl solution was used. Gas bubbles did not move freely through the aspirometer due to viscosity and drag effects of the saline solution.
The results obtained in the present study are not in agreement with those reported by Erben and Nadvornik (1963) and by Pierucci & Gherson (1969), who used an aqueous 20%wt NaCl solution and considered the dilution of the gases into the solution to be negligible5,22. Likewise, they are also discordant from the data obtained by Keil et al. (1980), who compared the use of distilled water with the use of saline-saturated dilution as liquid barriers and found no appreciable differences21. By contrast, we did find differences to be considered in both solutions. In this respect, it is important to highlight that our NaCl solution was also an acid solution and that we still did observe differences between this solution and distilled water.
A key outcome of the results from our investigations on stranded cetaceans is that the methodology was able to distinguish between putrefaction gases and embolism gases. As a matter of fact, embolic gases in fresh carcasses were predominantly composed of nitrogen, while gases from more autolyzed cetaceans showed higher quantities of CO2 and hydrogen. These results are in close agreement with previous reports in humans and laboratory animals4,5,6,7,8,9,10,11,12,13.
The gas composition results from the mass-stranded SWs show that bubbles did not resemble the gas composition of the intestine in any case. Gas from the coronary heart veins of one SW found stranded along the Italian coastline (SW 7) showed a composition that was very similar to the parameters defined for air embolism4,5. In this respect, the results of combined pathological and gas analysis investigations suggested that the aforementioned gas presence was most likely linked to the prolonged lateral recumbence of these very large and heavy whales on the beach. With little or no chance of thoracic breath movement, it is more than plausible that the SWs progressively suffered from hyperinflation of the passively congested lungs, with consequent rupture of alveolar walls, followed by interstitial and subpleural emphysema (as also confirmed by endothoracic hemorrhages), leading to pneumomediastinum and, most likely, to an associated pneumothorax and pneumopericardium condition15.
In contrast, the results of the gas bubble analysis, coupled with the necropsy findings of acute gas embolic lesions in the BW and acute and chronic gas embolic lesions in the Risso's dolphin, were both determined to be consistent with decompression-related excessive nitrogen supersaturation of tissues, potentially driving gas bubble formation and associated tissue injury. The higher content in nitrogen from the Risso's dolphin may be related to the chronicity of the lesions, enabling the washing out of metabolic gases (oxygen and nitrogen), while accumulating nitrogen over a much longer period of time via repeated cycles of nitrogen supersaturation associated with surface ascent or on-surface behavior. Oxygen is taken up by the surrounding tissues and CO2 is produced. As soon as CO2 reaches higher inner pressure than the pressures of the surrounding fluids, it will diffuse out and it will do so 20 times faster than oxygen and 40 times faster than nitrogen according to its diffusion coefficient. The high concentrations of nitrogen are a result of the washing out of metabolic gases.
Freezing of the spleen did not seem to affect later gas analyses. Possible loss of gas or atmospheric air entrance could have been expected because of changes in the tissue properties caused by freezing. Nitrogen is a relatively small gas with a mass weight of 28.01 u compare to 44.00 u of the CO2. If CO2 was present we should have detected it. On the other hand if atmospheric air (78% N2 and 21% O2) had entered into the gas filled cavities, higher oxygen content should have been found. However we don't recommend freezing complete animals when gas embolism is suspected. During the freezing and defrost period, putrefaction processes continue producing putrefactive gases that could mask the presence of previous embolism gases.
In conclusion, this study has experimentally tested and verified a protocol for the reliable and consistent collection, storage and analysis of gas from different body compartments in stranded cetaceans. By using these methods we have been able to show that gases in acute and chronic gas embolism-affected cetaceans that were minimally decomposed had high or very high nitrogen contents in bubbles. Further research is needed to expand the use of these methods more widely, including investigations of cetacean strandings both related and unrelated to suspected causal factors, such as proximity to high-intensity mid-frequency naval sonars.
Methods
Testing gas sampling and gas storage methodology
In these initial studies aimed at assessing normal gas contents in different vacutainers®, the gas contents of 5-mL additive-free vacutainers® were measured and compared with manually helium-purged 2-mL vacuum containers. The gas contents of the glass block-pressure syringe used for manual injection of gas into the gas chromatograph was also measured. The background air diffusion rate in different vacuum tubes (vacutainers®) was compared by injecting air (1 mL) into twenty 3.5 mL vacutainers® containing additives and into twenty 5.0 mL additive-free vacutainers®. All vacutainers® were kept upside-down at room temperature for 21 days and the gas content (area counts) was analyzed on storage days 1, 7, 14 and 21 (Fig. 1). The resultant graph was regression modeled and evaluated with the R2 coefficient. The most parsimonious model was chosen. Possible interactions between day and vacutainer type were also explored.
The best storage temperature for gas in vacutainers® was determined by the entry of a fixed amount of air (vacutainers® were allowed to equilibrate with atmospheric air using an open needle) into 60 vacutainers®, which were stored at different temperatures: room temperature (20–24°C), refrigerator (4°C) and freezer (−80°C). Air content was analyzed at days 1, 7, 14 and 21 after injection, with 5 replicates for each time and treatment. Data were statistically analyzed using a model of analysis of variance, with two factors of variation (temperature and day) and interactions between both factors. Multiple comparisons were carried out by means of the corresponding linear test.
In order to observe whether puncturing through the rubber could affect in later stage gas conservation inside the vacutainer®, 24 vacutainers® were sampled on two separate occasions. Possible differences in diffusion rate of different gases through the 5-mL additive-free vacutainer® were also evaluated at constant room temperature (21°C). Thirty vacutainers® were completely filled with atmospheric air and 5 replicates were analyzed each day from the same day of injection until the 5th day (Fig 2). This procedure was repeated with pure hydrogen and pure CO2 (Fig 2). In order to detect differences in gas levels respect to day zero, ratios with day zero were calculated for each day. Since oxygen and nitrogen were measured from the same tubes filled with atmospheric air, their ratio was treated in the same manner as each gas alone. In addition, average gas values for each day were also used to simulate a mixture of these gases.
Finally, to test whether vacutainers® were resistant to pressure changes and therefore suitable for transportation by airplane, 30 vacutainers® were completely filled with atmospheric air with 15 of them being also transported (round trip) in the passenger cabin of a turboprop aircraft. Duration of flight was of 35 min each way, with a maximum flight altitude of 3360 m. The levels of oxygen and nitrogen (airplane and control) were compared under two different experimental conditions using the Wilcoxon test for independent samples.
Insulin syringe testing
In order to properly sample bubbles in the cardiovascular system, disposable insulin syringes (BD Plastipak U-100 insulin) were used to sample bubbles and their contents were promptly injected into a vacutainer®. One new syringe and one new vacutainer® were used for each bubble. The accuracy of these syringes for gas volume measurements was studied by promptly injecting different atmospheric air volumes into vacutainers®. The air content injected from the insulin syringe into the vacutainer® was later analyzed by gas chromatography and statistical regression studies were performed.
The atmospheric air background of the insulin syringe was also analyzed. Syringes were used for the extraction of pure helium, which was then injected into the vacutainers®. Air content was analyzed and compared to that of intact vacutainers®. The levels of oxygen and nitrogen measured under the two experimental conditions were compared using the Wilcoxon test for independent samples.
Liquid barrier testing
If the carcass preservation status was fresh or very fresh (putrefaction grades 2 or 1, respectively), then a glass aspirometer (U201100896) was necessary to separate the gas from the blood found in the heart.
The aspirometer works by differences in pressure created by the vertical displacement of the simple gas flask. If this flask is moved upwards, the barrier liquid (distilled water) will move from this flask to the burette and from here all along the free rubber tube and the puncturing needle. This is the position in which the user must puncture the heart, since the entire system is filled with distilled water and atmospheric air pollution is not possible. It is also necessary in this case to fill the pericardial cavity with distilled water for the same purpose. Moving down the flask creates a negative pressure in the burette and in the free rubber tube, thus suctioning whatever is found inside the heart. By clamping the free rubber tube in this position, gas will ascend to the upper part of the burette and then the two physical phases will be separated. To collect the sample, it is only necessary to apply a vacutainer® and to open the stopcock. Therefore, this method uses a barrier liquid to separate the free gas from the blood and to avoid atmospheric air pollution of the sample.
Purified distilled water type 2, versus an aqueous solution of 20%wt NaCl and with a pH of 4, lowered by the addition of 1 M HCl droplets, were tested as barrier liquids. For both solutions, water purified with a combination of reverse osmosis, electrodeionization and ultraviolet light, using Elix 10 UV water purification system 230 V/50 Hz, were used. To compare both solutions, 1 mL of hydrogen and CO2 were introduced 10 times each through the rubber tubes and recovered with a 5-mL additive-free vacutainer® at the top of the gas burette with the aspirometer filled with distilled water. Its content was then compared with 10 vacutainers®, into which 1 mL of the corresponding gas had previously been injected. Both experiments were conducted under the same laboratory conditions. The same procedure was repeated for CO2 alone, with the aqueous 20%wt NaCl solution.
Gas analysis
Gas analysis was conducted by gas chromatography. Samples were injected manually into the analyzer (Varian 450-GC) with the use of a block-pressure syringe (Supelco A-2 series). The temperature of the injector was set at 230°C. This analyzer was equipped with a Varian CP7430 column composed of two different sub-columns in tandem: a (Q) PoraBOND Q column, for separation of CO2 and hydrocarbon compounds up to 4 carbons and a (M) Molsieve 5 A column, for separation of permanent gases (such as oxygen, nitrogen, argon, etc). To detect these compounds, it is necessary to have both a thermal-conductivity detector (TCD) and a flame-ionization detector (FID) disposed one after the other. The TCD is a universal detector for permanent gases. Its temperature was fixed at 80°C, while the filament temperature was 160°C. The FID is a selective hydrocarbon destructive detector. Because of its destructive nature, the FID must always be placed after the TCD. The temperature for the FID was fixed at 230°C. Samples were run for 25 minutes with an isothermal temperature of 45°C and an electronically controlled flux with a fixed pressure of 13.1 psi on the head column. Helium was used as the carrier gas.
Gas calculations
Since gases are temperature- and pressure-dependent, calibration curves were made for every gas involved in each session. Calibration curves were made using pure gases, except for oxygen and nitrogen, for which atmospheric air was used.
When samples were studied, the vacutainer's® background was corrected for by measuring it on blanks, which were always exposed to the same pressure, temperature and storage time conditions as the samples. The detection limit was set at  , where
, where  is the minimum detectable signal,
is the minimum detectable signal,  , is the average signal for a given gas in the blanks and
, is the average signal for a given gas in the blanks and  is the associated standard deviation18.
is the associated standard deviation18.
Statistical analysis
All data were analyzed using the ‘R’ data analysis software, version 2.11.1 (R Development Core Team, 2010). The numerical variables were summarized as means and standard deviations. Regression models were evaluated with the R2 coefficient. The most parsimonious model was chosen. Hypothesis testing was considered significant when the corresponding P-value was less than 0.05. The same consideration was made for analysis of variance studies and comparisons of two means. For small samples, the Wilcoxon test was applied.
Cetacean carcass dissection
Necropsy protocols and decomposition codes were established according to Kuiken and Hartman (1991)23. Any observed gas bubbles were sampled (e.g. those found in coronary heart veins), along with intestinal gases. If the carcass preservation status was fresh or very fresh (putrefaction grades 2 or 1, respectively), then the aspirometer filled with distilled water was used to separate the gas from the blood found in the heart after the pericardial sac had been filled with distilled water to avoid atmospheric air pollution.
Transport and storage of gas samples
Vacutainers® were kept upside-down at room temperature with one blank per sample, or a total of at least 3 blanks. Gas analysis was performed as soon as possible after sampling.
References
Jepson, P. D. et al. Gas-bubble lesions in stranded cetaceans - was sonar responsible for a spate of whale deaths after an atlantic military exercise? Nature 425, 575 (2003).
Fernandez, A. et al. “Gas and fat embolic syndrome” involving a mass stranding of beaked whales (family ziphiidae) exposed to anthropogenic sonar signals. Veterinary Pathology 42, 446 (2005).
Piantadosi, C. A. & Thalmann, E. D. Pathology: Whales, sonar and decompression sickness. Nature 428, 1 p following 716; discussion 2 p following 716 (2004).
Pierucci, G. & Gherson, G. Experimental study on gas embolism with special reference to the differentiation between embolic gas and putrefaction gas. Zacchia 4, 347 (1968).
Pierucci, G. & Gherson, G. Further contribution to the chemical diagnosis of gas embolism. The demonstration of hydrogen as an expression of “putrefactive component”. Zacchia 5, 595 (1969).
Bajanowski, T. et al. Proof of air embolism after exhumation. International Journal of Legal Medicine 112, 2 (1998).
Bert, P. La pression barometrique: Recherches de physiologie expérimentale. (Masson, Paris, 1878).
Harris, M. et al. Carbon dioxide as a facilitating agent in the initiation and growth of bubbles in animals decompressed to simulated altitudes. Journal of General Physiology 28, 225 (1945).
Ishiyama, A. Analysis of gas composition of intra vascular bubbles produced by decompression. Bulletin of Tokyo Medical and Dental University 30, 25 (1983).
Lillo, R. S., Maccallum, M. E., & Caldwell, J. M. Intravascular bubble composition in guinea-pigs a possible explanation for differences in decompression risk among different gases. Undersea Biomedical Research 19, 375 (1992).
Smith-Sivertsen, J., presented at the Proceedings of the Fith Symposium on Underwater Physiology, Washington, DC, 1976.
Armstrong, H. G., in Engineering Section Memorandum Report (Wright Field, Ohio, 1939).
Lawrence, C. Interpretation of gas in diving autopsies. South Pacific Underwater Medicine Society Journal 27, 228 (1997).
Dyrenfurth, F. Über den qualitativen und quantitativen nachweis von sauerstoff in lungenund darmgasen von leichen und seine anwendung bei der gerischtsärztlichen feststellung der atmung neugeborener. Dtsch. Z. ges. gerichtl. Med. 12, 23 (1928).
Mazzariol, S. et al. Sometimes sperm whales (physeter macrocephalus) cannot find their way back to the high seas: A multidisciplinary study on a mass stranding. PLoS ONE 6, e19417 (2011).
Lang, G. E., Mueller, R. G., & Hunt, P. K. Posible error resulting from use of “nitrogen-filled” vacutainers for blood-gas determinations. Clin Chem 19, 559 (1973).
Mueller, R. G. & Lang, G. E. Phase equilibrium of oxygen in nitrogen-filled vacutainers. Clinical Chemistry 19, 1198 (1973).
Kaiser, H. Die berechnung der nachweisempfindlichkeit. Spectrochimica Acta 3, 40 (1947).
Molloy, M. J., Latto, I. P., & Rosen, M. Analysis of nitrous-oxide concentrations in whole-blood - evaluation of an equilibration technique. British Journal of Anaesthesia 45, 556 (1973).
Knowles, T. P., Mullin, R. A., Hunter, J. A., & Douce, F. H. Effects of syringe material, sample storage time and temperature on blood gases and oxygen saturation in arterialized human blood samples. Respiratory Care 51, 732 (2006).
Keil, W. et al. Luftembolie oder fäulnisgas? Zur dianostik der cardialen luftembolie an der leiche. Beiträge zur Gerichtlichen Medizin 38, 395 (1980).
Erben, J. U. & Nádvornik, F. The quantitative demonstration of air embolism: In certain cases of fatal trauma. Journal of Forensic Medicine 10, (1963).
Kuiken, T. & García-Hartmann, M., presented at the 1st European Cetacean Society workshop on Cetacean Pathology., Leiden, Netherlands, 1991.
Acknowledgements
The authors would like to thank all colleagues who contributed to this work and, especially, the different stranding networks and governments: Canary Islands, Andalusia, United Kingdom and Italy, along with the hyperbaric medicine division of the NTNU (Norway) for its scientific contribution. This work was supported by the Spanish Ministry of Science and Innovation with two research projects: (AGL 2005-07947) and (CGL 2009/12663), as well as the Government of Canary Islands (DG Medio Natural). The Spanish Ministry of Education contributed with personal financial support (the University Professor Formation fellowship). The Risso's dolphin necropsy was conducted by the UK Cetacean Strandings Investigation Programme, funded by Defra and the Devolved Administrations (UK Government). The authors would like to thank Rod Penrose (Marine Environmental Monitoring/CSIP) for helping with the Risso's dolphin necropsy. Rod Penrose and Matt Perkins (Institute of Zoology) helped with Risso's dolphin gas collection and provided photos; we would also like to thank the Andalusian Government (Junta de Andalucía, Consejería de Medio Ambiente, Dirección General de Gestión del Medio Natural) for providing the photograph depicting the beaked whale on the beach.
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. Y.B. developed conceptually the methodology following O.G-D, A.F. and P.S. advices and performed fieldwork as well as laboratory validation and gas chromatography analyses. A.F. supervised the Project. A.F. and O.G-D contributed conceptually in its respective field: animal pathology and chemistry. P.S. conducted the statistical analyses and advised about the experimental designs from the statistical point of view. M.A, E.S. and S.S. contributed significantly to the fieldwork. P.J. contributed to edit the paper and did the Risso's dolphin necropsy, S.M. and G.D.G. organized the stranding response to the sperm whale mass stranding. Y.B., A.F., O.G-D and P.S. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
de Quirós, Y., González-Díaz, Ó., Saavedra, P. et al. Methodology for in situ gas sampling, transport and laboratory analysis of gases from stranded cetaceans. Sci Rep 1, 193 (2011). https://doi.org/10.1038/srep00193
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00193
This article is cited by
-
Establishment of a fish model to study gas-bubble lesions
Scientific Reports (2022)
-
Budd-Chiari-like pathology in dolphins
Scientific Reports (2022)
-
Multidisciplinary studies on a sick-leader syndrome-associated mass stranding of sperm whales (Physeter macrocephalus) along the Adriatic coast of Italy
Scientific Reports (2018)
-
Differentiation at autopsy between in vivo gas embolism and putrefaction using gas composition analysis
International Journal of Legal Medicine (2013)
-
First indication of gas embolism in a harbour porpoise (Phocoena phocoena) from German waters
European Journal of Wildlife Research (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.