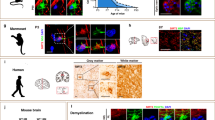
Abstract
Basic helix-loop-helix transcription factors Olig1 and Olig2 critically regulate oligodendrocyte development. Initially identified as a downstream effector of Olig1, an oligodendrocyte-specific zinc finger transcription repressor, Zfp488, cooperates with Olig2 function. Although Zfp488 is required for oligodendrocyte precursor formation and differentiation during embryonic development, its role in oligodendrogenesis of adult neural progenitor cells is not known. In this study, we tested whether Zfp488 could promote an oligodendrogenic fate in adult subventricular zone (SVZ) neural stem/progenitor cells (NSPCs). Using a cuprizone-induced demyelination model in mice, we examined the effect of retrovirus-mediated Zfp488 overexpression in SVZ NSPCs. Our results showed that Zfp488 efficiently promoted the differentiation of the SVZ NSPCs into mature oligodendrocytes in vivo. After cuprizone-induced demyelination injury, Zfp488-transduced mice also showed significant restoration of motor function to levels comparable to control mice. Together, these findings identify a previously unreported role for Zfp488 in adult oligodendrogenesis and functional remyelination after injury.
Similar content being viewed by others
Introduction
Demyelination in the central nervous system (CNS) is associated with a wide variety of disorders that either directly or indirectly induce oligodendrocyte (OL) injury or loss1. Although remyelination and recovery could occur under certain conditions 2, they are often limited and incomplete in acute injury or disease. Chronic demyelination of the CNS results in axonal degeneration and permanent loss of function 3. Although OL progenitors (OPs) are widely distributed in the adult CNS 4,5 and are often not directly affected by demyelination lesions 6,7, several studies point out that the prevailing pathological milieu in demyelination lesions can prevent OP proliferation 8,9 and their differentiation into mature OLs 10,11. Emerging studies indicate that neural stem/progenitor cells (NSPCs) in the neurogenic zones of the adult brain, including the subventricular zone (SVZ) and the hippocampal subgranular zone, can give rise to OPs and differentiate into OLs in conditions of myelin loss 12,13,14. However, the potential contribution of adult NSPCs to OL regeneration and remyelination still remains largely unclear.
Several stage-specific transcription factors have been identified to be responsible for coordinating OL differentiation 15,16,17. The basic helix-loop-helix (bHLH) transcription factors Olig1 and Olig2 have been demonstrated to play key roles in regulating OL development. Olig2 expression leads to the expression of other downstream factors, such as the homeobox factor Nkx2.218 and another bHLH protein Mash1 19 that promote differentiation to the early OL lineage. Olig2 also induces expression of the HMG-box transcription factor Sox10 that cooperates with Olig1 and is required for terminal OL differentiation 20,21. Other transcription factors such as Sox 9 are required for switching from a neuronal to a glial lineage 22. Sox 9 and Sox 10 in turn regulate the expression of platelet derived growth factor receptor α (PDGFRα) in OPs 23, while Nkx2.2 influences Sox10 and Olig2 expression 18. Moreover, a decrease in expression of transcriptional inhibitors, such as Hes5, Id2 and Id4, increases myelin gene expression and promotes terminal OL differentiation 24,25,26. Although not clearly demarcated in the literature, it has been suggested that Olig2 is the major transcription factor in OL lineage specification during development and that Olig1 may be important for remyelination and repair after demyelination lesions 27.
Further studies into the oligodendrogenic transcription network led to the identification of several OL-specific zinc finger proteins (Zfps) required for OL differentiation. Among these, Zfp191 is required for the myelinating function of OLs 28, Zfp Yin Yang 1 (YY1) regulates OP differentiation into mature OLs 29 and Zfp488 regulates OP formation and differentiation during embryonic development 30. Zfp488 was initially identified in a screen for differentially expressed genes in OLs from Olig1 knockout mice. Biochemical analysis revealed that it physically interacts with Olig2. In the chick neural tube, ectopic expression of Zfp488 induced precocious OL differentiation. Under conditions of constitutive Notch activation, Zfp488 expression also promotes precocious OP formation 30. In vivo characterization of Zfp488 in mice and its role in remyelination and repair remain to be studied.
In this manuscript, we examined whether Zfp488 induces oligodendrogenesis of SVZ NSPCs in the adult mouse brain after cuprizone-mediated myelin injury. We show that induced expression of Zfp488 in SVZ NSPCs significantly improved the number of OLs in the corpus callosum and translated to functional recovery. These observations clearly indicate that Zfp488 can promote oligodendrogenesis of SVZ NSPCs in the adult mouse brain. This new knowledge on Zfp488 has important implications in CNS myelin regeneration and repair after demyelinating diseases.
Results
Experimental design
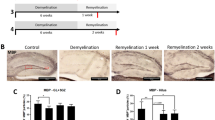
Zfp488 expression was under the control of a Moloney murine leukemia virus-long terminal repeat (MoMuLV-LTR; Figure 1A). Expression of Zfp488 was confirmed after a trial retroviral infection in 293T cells using both ZsGreen1 reporter fluorescence (Figure 1B) and western blots (Figure 1C). A well-characterized repressor element present in stem cells prevents expression of MoMuLV-LTR prior to their differentiation 31. By infecting mouse ES cells, we confirmed that there was no expression of Zsgreen1 and also found that expression was not observed even in embryoid bodies. This unique property allowed for the controlled or timed “switch on” of Zfp488 expression as progenitor cells start to differentiate but not during self-renewal within the SVZ NSPCs. In vivo, after delivery to the SVZ, the distribution of the ZsGreen1 positive cells was initially contained to the region immediately adjoining the ventricle on the ipsilateral side only (Figure 1D), consistent with in vitro observations. Moreover, the property of retroviruses to only integrate in proliferating cells allowed the specific targeting of proliferating SVZ progenitors at the specific injection locus. During the 5 weeks on a 0.2% cuprizone diet, mice progressively lost myelin, indicated by the loss of myelin basic protein (MBP) staining in the corpus callosum (Figure 1E). Mice on a cuprizone diet for 2 weeks underwent the procedure for intracerebral injection of Zfp488-expressing or control retroviruses into the SVZ. These mice continued on cuprizone diet for additional 3 weeks to reach the peak of demyelination and then removed from the cuprizone diet. Periodic analyses of histopathology and behavior were performed during the recovery phase. Recovery was examined for 6 additional weeks (Figure 1F).
Experimental design and retroviral vector construction.
(a) Schematic showing retroviral constructs, both empty vector (control) and with an insert coding for mouse Zfp488. After transduction, a Moloney murine leukemia virus (MMLV) long terminal repeats (LTR) drives Zfp488 expression. The vectors also express the fluorescent protein ZsGreen1 after an IRES to enable their identification and tracking. This promoter is not active in progenitor cells due to expression of a specific repressor element, but is transcribed once these cells start to differentiate. (b) ZsGreen1 fluorescence in Zfp488-overexpressing HEK 293T cells. (c) Western blots of Zfp488 retrovirus transduced HEK 293T lysate showing increased expression of Zfp488. (d) Representative Olig2 (red) immunolabeled image of the white matter adjoining the SVZ, 3 weeks after retrovirus injection, showing the distribution and integration of ZsGreen positive cells in mice that were not treated with cuprizone. (e) Images showing demyelination of the corpus callosum at different time points after cuprizone treatment. Sections were stained for myelin basic protein (MBP, red) and DAPI (blue). (f) For all experiments, two groups of mice were fed a 0.2% cuprizone diet for a period of 5 weeks. At two weeks, intracerebral injections into the SVZ of either control or Zfp488 expressing-retroviruses were carried out using a stereotaxic frame. After 5 weeks, mice in group I were used for behavior analysis and immunohistochemical characterization. Mice in group II were allowed to recover for an additional 6 weeks on normal diet without cuprizone. After the 6-week recovery period, tissues were collected to examine for the long-term survival of the retrovirus transduced-differentiated cells. Scale bar, 100 μm.
Zfp488 promotes differentiation of SVZ progenitor cells to OLs
In order to examine whether forced expression of Zfp488 in differentiating SVZ NSPCs would direct an oligodendrogenic fate after cuprizone-induced demyelination, we examined mice that underwent an intracerebral injection of retroviral Zfp488-ZsGreen1 in the SVZ in comparison to control ZsGreen1 injected mice. At the peak of demyelination (5 weeks) we examined histological sections of the brain for the co-localization of ZsGreen1 (retrovirus infected differentiated cells derived from SVZ progenitor cells) and Olig2. In Zfp488 retrovirus injected mice, ZsGreen1 expressing cells were abundant within the corpus callosum and co-labeled with Olig2 at a significantly higher rate (68 ± 8.6 %; p = 0.003; Figure 2A and 2C) compared to the control retrovirus injected mice (25 ± 5 %; Figure 2B and 2C). Zfp488 expressing cells were observed to have migrated the entire length of the corpus callosum with the number of positive cells progressively reducing as the corpus callosum tapered. We found that in cuprizone induced demyelination, SVZ cells did not adhere to the rostral migratory stream and in both groups, we observed only rare ZsGreen1 positive cells within regions of the olfactory bulb (data not shown). A striking observation was that Zfp488-ZsGreen1 expressing cells were always exclusively restricted to the white matter of the corpus callosum and were almost never found in other regions of the brain. In stark contrast, control cells were more randomly distributed in regions of both the white and grey matter and had predominantly integrated into the neuronal circuitry adjoining the corpus callosum white matter (labeled with HuC/HuD in figure 4).
Zfp488 directs the differentiation of SVZ progenitor cells into mature oligodendrocytes at the peak of cuprizone-induced demyelination.
Mice on a 0.2% cuprizone diet for 2 weeks were injected with either control or Zfp488 expressing retroviruses into the SVZ. After 5 weeks in the cuprizone diet, brain sections were evaluated for the differentiation status of the virus transduced SVZ stem/progenitor cell population. (a) Representative image of the Zfp488 virus transduced SVZ-derived cells in the white matter of the corpus callosum. Higher magnification panel show that cells transduced with the Zfp488 retrovirus differentiated into oligodendrocytes and were positive for Olig2. (b) Representative image of the control (ZsGreen1) virus transduced SVZ-derived cells in the white matter of the corpus callosum. Higher magnification panel show that cells transduced with the control retrovirus did not differentiate into oligodendrocytes and were negative for Olig2. (c) Percentage of virus-transduced SVZ-derived cells that expressed Olig2. The number of ZsGreen1 positive cells that were positive for Olig2 was significantly higher in mice overexpressing Zfp488 (n=4) compared to controls (n=5, p=0.003). (d) SVZ-derived cells in the corpus callosum at 5 weeks of cuprizone-induced demyelination. Representative images show that Zfp488-expressing cells were also positive for the oligodendroglial marker Sox10 compared to control cells that were negative for Sox10. (e) SVZ-derived cells in the corpus callosum at 5 weeks of cuprizone-induced demyelination. Representative images show that Zfp488-expressing cells were also positive for the mature oligodendrocyte marker GSTπ compared to control cells that were negative for GSTπ. Scale bars, 100 μm and 50 μm for the lower and higher magnification images, respectively.
Zfp488 overexpressing SVZ cells did not differentiate into neurons or astrocytes.
Mice on a 0.2% cuprizone diet for 2 weeks were injected with either control or Zfp488 expressing retrovirus into the SVZ. After 5 weeks in the cuprizone diet and 6 weeks recovery on normal chow, brain sections were evaluated for the SVZ cells that had differentiated into neuronal and astrocytic populations. (a) At 5 weeks, a population of control virus expressing SVZ-derived cells in the corpus callosum co-localized with the neuronal marker HuC/HuD (arrows); however, Zfp488 overexpressing cells did not differentiate into neurons and were negative for HuC/HuD. (b) At the same 5 week time point, a population of control virus expressing SVZ-derived cells in the corpus callosum co-localized with the astrocyte marker S100β (arrows); however, Zfp488 over expressing cells did not differentiate into mature astrocytes and were negative for S100β. (c) After 6 weeks of recovery from cuprizone-induced demyelination, both control and Zfp488 overexpressing cells were restricted to the white matter tract and were negative for the neuronal marker HuC/HuD. (d) At the same 6 weeks of recovery, most control virus expressing cells co-localized with the astrocyte marker S100β (arrows); however, Zfp488 overexpressing cells did not give rise to an astrocyte population and were negative for S100β. Scale bars, 50 μm.
In addition to Olig2, Zfp488-ZsGreen1 expressing cells also co-labeled with another OL lineage marker SOX10 and the mature OL marker GST-π while the significantly fewer control cells observed in the corpus callosum did not show such co-localization (Figure 2D and E).
Zfp488-expressing OLs survive long term in the remyelinated corpus callosum
In order to examine whether the Zfp488-infected OLs that migrated to the corpus callosum from the SVZ at the peak of demyelination were able to integrate and survive in the white matter, we examined mice, 6 weeks after removal from the cuprizone diet. We observed that Zfp488-ZsGreen1 expressing cells were still abundant in the corpus callosum. These Zfp488-ZsGreen1 expressing cells co-labeled for the oligodendrocyte lineage marker Olig2 at a significantly higher rate (60 ± 3.5 %; p<0.001; Figure 3A and 3C) compared to control-ZsGreen1 expressing cells (15 ± 2.3 %; Figure 3B and 3C). The cell numbers seen after 6 weeks of recovery were similar to that observed at the peak of demyelination suggesting that most if not all cells were able to survive long-term after remyelination and recovery in these mice. In addition, similar to that observed at the peak of demyelination, Zfp488-Zsgreen1 expressing cells expressed SOX10 and GST-π while the few control cells that remained in the corpus callosum did not show any co-localization (Figure 3D and E).
Mature oligodendrocytes derived from Zfp488 overexpressing SVZ progenitor cells survive after complete recovery from cuprizone-induced demyelination.
Mice on a 0.2% cuprizone diet for 2 weeks were injected with either control or Zfp488 expressing retrovirus into the SVZ. After cuprizone treatment for 5 weeks, mice were allowed to recover and remyelinate for 6 weeks on normal chow. After 5 weeks in the cuprizone diet, brain sections were evaluated for the differentiation status of the virus-transduced SVZ progenitor cell population. (a) Representative image of the Zfp488 virus transduced SVZ-derived cells in the white matter of the corpus callosum. Higher magnification panel show that cells transduced with the Zfp488 retrovirus differentiated into oligodendrocytes and were positive for Olig2. (b) Representative image of the control (ZsGreen1) virus transduced cells in the white matter of the corpus callosum. Higher magnification panel show that cells transduced with the control retrovirus did not differentiate into oligodendrocytes and were negative for Olig2. (c) Percentage of virus-transduced SVZ-derived cells that express Olig2. The number of ZsGreen1 positive cells that were positive for Olig2 was significantly higher in mice expressing Zfp488 (n=5) compared to controls (n=4, p<0.001). (d) SVZ-derived cells in the corpus callosum after 5 weeks of cuprizone-induced demyelination followed by 6 weeks of recovery. Representative images showed that Zfp488 expressing cells were also positive for the oligodendroglial marker Sox10 compared to control cells that were negative for Sox10. (e) SVZ-derived cells in the corpus callosum after 5 weeks of cuprizone-induced demyelination followed by 6 weeks of recovery. Representative images showed that Zfp488-expressing cells were also positive for the mature oligodendrocyte marker GSTπ compared to control cells that were negative for GSTπ. Scale bars, 100 μm and 50 μm for the lower and higher magnification images, respectively.
Zfp488-induced differentiation is predominantly oligodendrogenic
As SVZ NSPCs are capable of differentiation into all the three major cell types in the brain, neurons, oligodendrocytes and astrocytes 32,33, we examined the lineage commitment of Zfp488-ZsGreen1 expressing SVZ cells compared to control cells. Using specific markers for neurons (HuC/HuD) and astrocytes (S100β), we examined for co-localization of Zfp488-ZsGreen1 and control cells with these markers. We observed that Zfp488-ZsGreen1 expressing cells were mostly confined to the corpus callosum and rarely co-expressed markers for neurons or astrocytes at either the peak of demyelination (Figure 4A and B) or after the 6-week recovery period (Figure 4C and D) suggesting that their differentiation was predominantly oligodendrogenic. In contrast, control cells that were more randomly distributed in the brain extensively co-labeled with markers for neurons and astrocytes at both the peak of demyelination and 6 weeks of recovery (Figure 4) suggesting that their lineage was randomized during the recovery phase. These observations along with the distribution patterns of SVZ-derived cells in the brain provides additional evidence that Zfp488 expression in SVZ cells targets them to the white matter of the corpus callosum and are destined to an OL fate.
Zfp488-induced remyelination results in improved functional recovery
Next, we examined whether enhanced oligodendrogenesis contributed by directing the differentiation of adult SVZ NSPCs with Zfp488 could be measured by functional recovery parameters. In order to test functional end points, we assessed the performance of Zfp488 injected and control groups of mice on an accelerating rotarod test and subsequently a grip strength test. We performed these tests during the early recovery period immediately after they were removed from a cuprizone diet after 5 weeks for 3 days. We also assessed an age matched sham group of mice that did not receive the cuprizone diet. Initial results from these trials were consistent with the literature in that cuprizone-treated mice performed poorly in these motor tests compared to sham 34 (Figure 5). Evaluating functional recovery in comparison to the sham group, we found that at Day 3, Zfp488 recovered to a level that it was no longer different from the sham (p = 0.84); whereas, performance of the cuprizone control group remained significantly lower (p = 0.02). Within treatment groups, we also recorded that the Zfp488 group had significantly improved abilities of motor control on the accelerating rotarod that was statistically significant compared to control group (p = 0.02). This finding suggested that a significant improvement in motor function occurred in the Zfp488 group. This result was strengthened by the more stringent analysis that mean total run time calculated for the entire 3 days was also significantly higher for the Zfp488 group compared to control group (p = 0.03; n = 20/group; Figure 5A).
In vivo overexpression of Zfp488 in SVZ progenitor cells improved functional recovery in mice after cuprizone-induced demyelination.
(a) Cuprizone-treated mice induced to overexpress Zfp488 in SVZ cells (Zfp-cz, n=20) were examined for motor function and compared to control virus injected mice (Con-cz, n=20) and sham mice on normal chow (Sham, n=19). The mice induced to over express Zfp488 in SVZ stem cells (Zfp-cz) stayed significantly longer on the rod compared to control (Con-cz) mice on Day 3. This difference was also apparent on the mean run time for all 3 days. In addition, day 3 of testing and mean run time shows that the motor control of the Zfp488 overexpressing cells matched that of the sham mice (* p=0.05 vs Sham, *** p=0.01 vs Sham, ∧∧ p=0.02 vs Con-cz, ∧ p=0.03 vs Con-cz). (b) Forelimb grip test on both groups of cuprizone-treated mice (Zfp-cz and Con-cz) showed significantly lower scores compared to the group of sham mice. However, there were no statistically significant differences between the Zfp-cz and the Con-cz group (** p=0.02 vs Sham) (c) Hindlimb grip test on both groups of cuprizone-treated mice (Zfp-cz and Con-cz) showed significantly lower scores compared with the group of sham mice. However, there were no statistically significant differences between the Con-cz and Zfp-cz groups (***p=0.01 vs Sham).
For a measure of peripheral neuromuscular function, we examined both forelimb and hindlimb grip strength as a response not directly associated with CNS remyelination and recovery. In all three trials (Days 1–3), the performance of both Zfp488 and control groups that received cuprizone was at a level lower than the sham group. As expected, we did not detect any significant improvements in Zfp488 group compared to control group in both forelimb and hindlimb grip strength tests (n = 19–20; Figure 5B and C). These data suggest that the recovery observed in the rotarod performance is associated with enhanced remyelination in the corpus callosum of the Zfp488 group of mice.
Discussion
Zfp488 was recently reported as a highly conserved OL-specific transcription factor with functions potentially interwoven with other factors critical for oligodendrogenesis 30. In this study, we examined the specific function of Zfp488 in OL development and differentiation in vivo after timed overexpression in SVZ NSPCs. By using a cuprizone-induced demyelination mouse model, we demonstrate that Zfp488 could significantly promote an oligodendrogenic fate of differentiating SVZ NSPCs and enhance functional recovery.
SVZ NSPCs have been used as a tool to scrutinize factors that promote oligodendrocyte fate specification and remyelination in vivo in several studies 35,36,37. The SVZ is a major source of NSPCs in the adult mouse brain. These NSPCs correspond to a population of astrocyte-like cells (type B cells) that give rise to actively proliferating transit amplifying type C cells 38. Under physiological conditions, SVZ type C cells contribute to formation of neuroblasts (type A cells) that migrate via the rostral migratory stream (RMS) to the olfactory bulb and replace interneurons 39. Under demyelinating CNS pathologies, SVZ NSPCs have been shown to be able to proliferate and generate both parenchymal OPs and mature OLs, most of which are found integrated in the corpus callosum 12,13,14,40.
Several studies have examined the different cues required to direct SVZ NSPC differentiation into OPs and subsequently to OLs 41,42. In a lysolecithin-induced demyelination model, intraventricular infusion of epidermal growth factor dramatically promoted proliferation, migration and differentiation of SVZ NSPCs into OLs 43. In another study adenoviral expression of leukemia inhibitory factor significantly promoted SVZ NSPC self-renewal 44. Similarly, intraventricular infusion of the BMP inhibitor Noggin also increased the number of Olig2-positive OLs in mice after cuprizone-induced demyelination 35. In addition to the use of diffusible factors, several experiments have explored intrinsic transcriptional fate determinants that regulate OL development and differentiation from SVZ NSPCs 45. Inducible overexpression of Olig2 and Olig1 have been identified to play crucial roles in determining an OL fate during SVZ NSPC differentiation in vivo 46.
In this study, we examined the oligodendrogenic effect of timed Zfp488 overexpression in differentiating SVZ NSPCs in a cuprizone-induced demyelination model. The MMLV LTR element driving Zfp488 expression was not constitutively active in SVZ NSPCs but was restricted to only differentiating cells migrating out of this region. In addition, the use of a chemical-induced demyelination model in mice had unique experimental advantages in that the SVZ is in close proximity to the corpus callosum and cuprizone treatment can be terminated to allow a more direct analysis of different cellular contributions to remyelination. In cuprizone treatment, myelin loss has been shown to be specifically associated with the loss of mature OLs but not their progenitors 47,48,49. Moreover, treating with cuprizone does not have any effect on the peripheral nerves 50. Unlike the autoimmune demyelination pathology seen in multiple sclerosis or experimental autoimmune encephalomyelitis, cuprizone-induced demyelination lacks secondary complications brought about by blood brain barrier breakdown and therefore does not adversely affect the CNS microenvironment allowing for near complete remyelination. This feature makes it ideal to examine OL precursor formation and differentiation from SVZ NSCs in the absence of secondary pathological effects 27. The absence of immune cells in this model also supports increased proliferation of OPs 27. Moreover, functional correlates for longitudinal analysis allow for the evaluation and comparison of demyelination and remyelination in detail under different experimental conditions 51.
OL differentiation after demyelination is dependent on both extrinsic and intrinsic factors 3. Different factors that promote OL differentiation of NSPCs have been identified to be upregulated in and around the SVZ. For example: p75(NTR), the low-affinity neurotrophin receptor, has been shown to be upregulated by oligodendroglial progenitors adjacent to the subventricular zone in response to demyelination 52. More recently, chordin, a factor that could redirect GAD65 and doublecortin positive NSPCs to an glial rather than neuronal lineage has also been reported to be responsible for generating new OLs in the corpus callosum after demyelination 12. In this study, mice were injected with a Zfp488 retrovirus after two weeks on a cuprizone diet when there is a high rate of OPC proliferation at onset of demyelination 53. At the peak of demyelination, we located the Zfp488-ZsGreen1 expressing OLs that were confined to the corpus callosum white matter tract. We observed that Zfp488 over expressing SVZ NSPCs differentiated readily into OLs compared to controls. At the end of the recovery period, Zfp488 overexpressing OLs had migrated along the entire corpus callosum, showing integration and survival in the newly generated white matter. It is possible that Zfp488 overexpression might lead to increased proliferation of the progenitors targeted by the retrovirus or cause cell death of other neuronal lineages. However previous in vitro studies definitively indicated that Zfp488 overexpression directs differentiation of progenitors to oligodendrocyte precursors 30. We also observed an identical number ZsGreen1 expressing cells in the corpus callosum white matter and adjoining grey matter in both Zfp488 transduced and control virus transduced cells respectively, further confirming that there was neither cell death nor proliferative effects as a result of Zfp488 expression. Although these cells labeled for different markers the transduction efficiency was similar in both conditions. The deficiency of ZsGreen1 positive cell migration into the olfactory bulb through the rostral migratory stream could be explained by the extreme demyelinating milieu 54 and poor oligodendrogenesis in the olfactory bulb 55, of this cuprizone demyelination mouse model. In addition, Zfp488 transduced Olig2 positive cells were always localized to the white matter tract at both the peak of demyelination and 6 weeks after recovery (i.e. 11 weeks after starting the cuprizone diet) suggesting that the differentiation fate of these cells remained oligodendroglial rather than astrocytic. In contrast the control virus transduced cells differentiated into astrocytes (S100β positive) and neurons (HuC/HuD positive) as expected of the multipotent progenitors targeted by the virus. The absence of S100β staining on the Zfp488 transduced cells and their strict white matter localization is further confirmation that these cells do not differentiate to astrocytes.
These histochemical findings were in strong correlation with improvement in motor function observed in the Zfp488 injected mice. Cuprizone-induced demyelination is known to impair corpus callosum-associated bilateral sensorimotor coordination in mice 34,51. After 5 weeks on a cuprizone diet (peak of demyelination), deficits were evident in the frequency of falls and the running time on the rotarod, consistent with corpus callosum demyelination. Our observations clearly showed that the Zfp488 group performed significantly better in run times compared to controls. Remarkably at day 3, the performance of the Zfp488 group was similar to sham group that did not receive a cuprizone diet suggesting a significant recovery in this group. In order to explore the specificity of the corpus callosum demyelination and this recovery effect, we also analyzed forelimb and hindlimb grip strength, indicators of peripheral neuromuscular function 56. We did not find any differences between the Zfp488 group and control group in this functional test demonstrating that the increased motor coordination observed in the Zfp488 group was indeed due to specific CNS effects that involve remyelination in the corpus callosum. Although astrocytes and neurons have been reported to influence myelination 57,58,59 the oligodendrogenic effect of Zfp488 and the subsequent increase in myelination superseded any endogenous factors as evidenced by the absence of functional gain in the control virus injected mice.
Together, the immunohistochemical and functional data clearly support the conclusion that Zfp488 overexpression in differentiating NSPCs directs them to an OL fate. Attempts to localize Zfp488 protein expression during CNS OP and OL development in mice were futile as none of the commercially available antibodies could faithfully identify Zfp488 in tissue sections. This issue has also been encountered in a previous study characterizing Zfp488 in which, only in situ hybridization for Zfp488 mRNA localization could be reported 30. Nevertheless, in the present study, ZsGreen1 reporter fluorescence reliably reflected the expression of Zfp488 protein. Previous co-immunoprecipitation studies using recombinant Zfp488 and Olig2 showed a physical interaction between these two proteins in vitro 30, suggesting the involvement of Zfp488 in the transcriptional network of Olig2 that is critical for OP and OL differentiation 45,46,60. Moreover, Zfp488 expression was dramatically reduced in Olig1 knockout mice and was shown to be regulated by Olig1 30. Observations in this manuscript are consistent with these reports in that a functional integration of Zfp488 with Olig1 and Olig2 could bring about directed differentiation of SVZ NSPCs to OLs.
Specific mechanisms that regulate OL development and differentiation are not completely understood and continue to remain a topic of active investigation. Advancing current knowledge, our results clearly demonstrate a role for Zfp488 in oligodendrogenesis. These results not only provide insights into the transcriptional regulation of OL development but also identify a candidate gene to promote oligodendrogenesis from SVZ NSPCs in vivo after demyelination.
Methods
Reagents and animals
All chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO), unless otherwise specified. Primary antibodies against myelin basic protein (MBP) was from Novus Biologicals (Littleton, CO), Olig2 was from R&D systems (Minneapolis, MN), S100β was from Sigma-Aldrich, GST-π was from BD biosciences (San Jose, CA), HuC/HuD was from Invitrogen (Carlsbad, CA), Sox10 was from Santa Cruz Biotechnology (Santa Cruz, CA) and Zfp488 was from LifeSpan Biosciences (Seattle, WA) and Santa Cruz Biotechnology (S14 and C14; Santa Cruz, CA). Fluorophore conjugated secondary antibodies were from Invitrogen. All mice used in these experiments were purchased from Charles River laboratories (Wilmington, MA). All animals were maintained in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and all experiments were performed with approved protocols from the Institutional Animal Care and Use Committee of the University of California, Davis.
Plasmids and retrovirus production
The mouse Zfp488 cDNA was a gift from Dr Richard Lu, University of Texas Southwestern Medical Center, Dallas, TX, USA. The Zfp488 open reading frame was cloned into the pRetroX-IRES-ZsGreen1 vector from Clontech laboratories (Mountain View, CA). The empty vector expressing ZsGreen1 was used as a control. Replication incompetent retroviruses were produced from pRetroX-Zfp488-IRES-ZsGreen and pRetroX-IRES-ZsGreen1 (control) at the Gene Transfer Vector Core, University of Iowa (Iowa City, IA). Titers for the concentrated stocks were obtained for both the viruses and were >2×108 TU/ml.
Cuprizone-induced demyelination
CNS demyelination was induced by cuprizone in mice by following an established protocol 34. A diet containing 0.2% cuprizone (w/w) was custom ordered from Lab Diet (Richmond, IN). Eight-week-old C57BL/6 male mice were fed either cuprizone or control pellet diets ad libitum for 5 weeks to induce demyelination. Pellets were changed once every two days and the weights of mice were recorded every week. After 5 weeks, mice were returned to a regular diet for analyzing remyelination and recovery.
Stereotaxic retrovirus injection to the subventricular zone
Mice fed 0.2% cuprizone for 2 weeks were used for all intracerebral injections. To perform the procedure, mice were positioned on a stereotaxic device (David Kopf, Tujunga, CA) under continuous isoflurane inhalation anesthesia. Target coordinates were optimized with the following instrument settings: The head was immobilized with the mouse adapter (x axis at 37 mm, y axis at −15 mm, the dorsal ventral tilt at 15° and the coronal angle at 0°) and ear bars. The anterior part of the SVZ was targeted with the coordinates: 2 mm anterior to bregma, 1.5 lateral to midline and 2.5 mm below dura mater. Two microliters of the retrovirus (2×108 TU/ml) was injected using a Nanoject II microinjector (Drummond Scientific Company, Broomall, PA) into the SVZ of the right hemisphere. The injection pipette was left in place for 5 minutes to prevent back flow before retraction.
Western blotting
293T cells transduced with control or Zfp488-expressing retroviruses were homogenized in ice-cold lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate, 2 mM EDTA (pH 7.4) and a proteinase inhibitor mixture. After clarifying by centrifuging at 14,000×g at 4°C, protein concentration in the extracts was determined by BCA protein assay kit (Pierce, Rockford, IL). Extracts containing 30 μg total protein were denatured with SDS sample buffer and separated by 4–15% SDS-PAGE (Bio-Rad Laboratories, Hercules, CA). Proteins were transferred onto nitrocellulose membranes that were then incubated with blocking solution (5% nonfat dried milk dissolved in TBST buffer containing 10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20) for 1 hr at room temperature and incubated with an anti-rabbit primary antibody against Zfp488 (1∶500, Lifespan Biosciences, Seattle, WA) or an anti-mouse primary antibody against GAPDH (1∶2000) at 4°C overnight. Membranes were washed three times with TBST buffer and incubated with the appropriate HRP-conjugated secondary antibodies (1∶2000 dilution) for 1 hr followed by washing three times. Signal detection was performed with a chemiluminescence kit (Pierce, Rockford, IL).
Immunohistochemistry
Brains were collected after perfusing the mice with 4% formaldehyde (w/v) (Electron Microscopy Sciences, Hatfield, PA). After 1 hour of post-fixation, tissues were placed in 20% sucrose solution (w/v) for 2 days. The samples were then embedded in OCT compound (Sakura Finetek, Torrance, CA) and frozen in liquid nitrogen-cooled isopentane. Ten micron sections were prepared from the frozen blocks in a cryotome and stored at −80°C. For staining, the non-specific epitopes were first blocked and the cells were permeabilized using 10% normal goat serum and 0.3% triton-X-100 in PBS at room temperature. Samples were subsequently incubated with primary antibodies overnight at 4°C. Slides were then washed with PBS and incubated with fluorophore-conjugated secondary antibodies at 1∶200 dilutions for one hour at room temperature. Nuclear counterstaining was carried out using DAPI (300nM, Invitrogen) before mounting the slides using ProlongGold mounting medium (Invitrogen). Images were acquired in a Nikon A1 confocal microscope and analyzed. To enumerate and identify the number of virus transduced cells, all ZsGreen1 expressing cells co-labeled with different differentiation markers were counted from serial sections 100 μm apart. An average of 100 cells were counted for each animal, with n = 5 per treatment.
Behavioral analysis
Animals were divided into three groups (n = 19–20 mice/group): (1) sham surgery mice (0% cuprizone feeding regimen through out the study), (2) ZsGreen1 control injections (0.2% cuprizone regimen) and (3) Zfp488 injections (0.2% cuprizone regimen). All tests described below were conducted at the end of the 5-week period on cuprizone diet. A trained observer blinded to the different treatment groups carried out all behavioral assessments.
Rota rod analysis: An accelerating Rota rod analysis was carried out on a Rotamex instrument (Columbus instruments, Columbus, OH) for three consecutive days. The mice underwent an initial training day (Day 1) on followed by two days (Day 2 and Day 3) with three test sessions each. Each session lasted 360 seconds. The initial speed of the rotating rod was set at 4 rpm and increased by 1 rpm every 10 seconds to a maximum of 40 rpm. The time each animal stayed on the rod was recorded.
Grip strength test: Grip strength was measured by strain gauge (Grip Strength Meter, Columbus Instruments). Fore- and hind-grip were measured separately on three successive trials at the end of cuprizone treatment and averaged.
Statistical analysis
Data were analyzed using one-way analysis (ANOVA) using the SPSS software (Chicago, IL). A Tukey post hoc test for the comparison of multiple groups was used when appropriate. In all figures and text, data are represented as mean ± SEM. Significant effects were identified at p<0.05.
References
Ben-Hur, T. & Goldman, S.A. Prospects of cell therapy for disorders of myelin. Ann N Y Acad Sci 1142, 218–249 (2008).
Duncan, I.D., Brower, A., Kondo, Y., Curlee, J.F., Jr & Schultz, R.D. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A 106, 6832–6836 (2009).
Franklin, R.J. & Ffrench-Constant, C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9, 839–855 (2008).
Nishiyama, A., Lin, X.H., Giese, N., Heldin, C.H. & Stallcup, W.B. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43, 299–314 (1996).
Reynolds, R. & Hardy, R. Oligodendroglial progenitors labeled with the O4 antibody persist in the adult rat cerebral cortex in vivo. J Neurosci Res 47, 455–470 (1997).
Sim, F.J., Zhao, C., Penderis, J. & Franklin, R.J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22, 2451–2459 (2002).
Chari, D.M. & Blakemore, W.F. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia 37, 307–313 (2002).
John, G.R. et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med 8, 1115–1121 (2002).
Mi, S. et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat Neurosci 8, 745–751 (2005).
Kotter, M.R., Li, W.W., Zhao, C. & Franklin, R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci 26, 328–332 (2006).
Kuhlmann, T. et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain 131, 1749–1758 (2008).
Jablonska, B. et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci 13, 541–550 (2010).
Menn, B. et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26, 7907–7918 (2006).
Nait-Oumesmar, B. et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci 11, 4357–4366 (1999).
Chandran, S. & Compston, A. Neural stem cells as a potential source of oligodendrocytes for myelin repair. J Neurol Sci 233, 179–181 (2005).
Nicolay, D.J., Doucette, J.R. & Nazarali, A.J. Transcriptional control of oligodendrogenesis. Glia 55, 1287–1299 (2007).
Wegner, M. A matter of identity: transcriptional control in oligodendrocytes. J Mol Neurosci 35, 3–12 (2008).
Zhou, Q., Choi, G. & Anderson, D.J. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31, 791–807 (2001).
Parras, C.M. et al. The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci 27, 4233–4242 (2007).
Stolt, C.C. et al. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16, 165–170 (2002).
Li, H., Lu, Y., Smith, H.K. & Richardson, W.D. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J Neurosci 27, 14375–14382 (2007).
Stolt, C.C. et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17, 1677–1689 (2003).
Finzsch, M., Stolt, C.C., Lommes, P. & Wegner, M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development 135, 637–646 (2008).
Kondo, T. & Raff, M. The Id4 HLH protein and the timing of oligodendrocyte differentiation. EMBO J 19, 1998–2007 (2000).
Liu, A. et al. A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J 25, 4833–4842 (2006).
Wang, S., Sdrulla, A., Johnson, J.E., Yokota, Y. & Barres, B.A. A role for the helix-loop-helix protein Id2 in the control of oligodendrocyte development. Neuron 29, 603–614 (2001).
Arnett, H.A. et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4, 1116–1122 (2001).
Howng, S.Y. et al. ZFP191 is required by oligodendrocytes for CNS myelination. Genes Dev 24, 301–311 (2010).
He, Y. et al. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron 55, 217–230 (2007).
Wang, S.Z. et al. An oligodendrocyte-specific zinc-finger transcription regulator cooperates with Olig2 to promote oligodendrocyte differentiation. Development 133, 3389–3398 (2006).
Dodge, J.E., Ramsahoye, B.H., Wo, Z.G., Okano, M. & Li, E. De novo methylation of MMLV provirus in embryonic stem cells: CpG versus non-CpG methylation. Gene 289, 41–48 (2002).
Levison, S.W. & Goldman, J.E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 10, 201–212 (1993).
Lois, C. & Alvarez-Buylla, A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc Natl Acad Sci U S A 90, 2074–2077 (1993).
Franco-Pons, N., Torrente, M., Colomina, M.T. & Vilella, E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett 169, 205–213 (2007).
Cate, H.S. et al. Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. Journal of neurochemistry 115, 11–22 (2010).
Franco, P.G., Silvestroff, L., Soto, E.F. & Pasquini, J.M. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp Neurol 212, 458–467 (2008).
Mamber, C., Verhaagen, J. & Hol, E.M. In vivo targeting of subventricular zone astrocytes. Prog Neurobiol 92, 19–32 (2010).
Doetsch, F., Caille, I., Lim, D.A., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Lois, C. & Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145–1148 (1994).
Picard-Riera, N. et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99, 13211–13216 (2002).
Rogister, B., Ben-Hur, T. & Dubois-Dalcq, M. From neural stem cells to myelinating oligodendrocytes. Mol Cell Neurosci 14, 287–300 (1999).
Chong, S.Y. & Chan, J.R. Tapping into the glial reservoir: cells committed to remaining uncommitted. J Cell Biol 188, 305–312 (2010).
Gonzalez-Perez, O., Romero-Rodriguez, R., Soriano-Navarro, M., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 27, 2032–2043 (2009).
Bauer, S. & Patterson, P.H. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci 26, 12089–12099 (2006).
Marshall, C.A., Novitch, B.G. & Goldman, J.E. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci 25, 7289–7298 (2005).
Maire, C.L., Wegener, A., Kerninon, C. & Nait Oumesmar, B. Gain-of-function of Olig transcription factors enhances oligodendrogenesis and myelination. Stem Cells 28, 1611–1622 (2010).
Jurevics, H. et al. Alterations in metabolism and gene expression in brain regions during cuprizone-induced demyelination and remyelination. J Neurochem 82, 126–136 (2002).
Matsushima, G.K. & Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 11, 107–116 (2001).
Morell, P. et al. Gene expression in brain during cuprizone-induced demyelination and remyelination. Mol Cell Neurosci 12, 220–227 (1998).
Komoly, S. Experimental demyelination caused by primary oligodendrocyte dystrophy. Regional distribution of the lesions in the nervous system of mice [corrected].Ideggyogy Sz 58, 40–43 (2005).
Hibbits, N., Pannu, R., John Wu, T. & Armstrong, R.C. Cuprizone demyelination of the corpus callosum in mice correlates with altered social interaction and impaired bilateral sensorimotor coordination. ASN Neuro 1 (2009).
Petratos, S. et al. Expression of the low-affinity neurotrophin receptor, p75(NTR), is upregulated by oligodendroglial progenitors adjacent to the subventricular zone in response to demyelination. Glia 48, 64–75 (2004).
Mason, J.L. et al. Mature oligodendrocyte apoptosis precedes IGF-1 production and oligodendrocyte progenitor accumulation and differentiation during demyelination/remyelination. J Neurosci Res 61, 251–262 (2000).
McTigue, D.M. & Tripathi, R.B. The life, death and replacement of oligodendrocytes in the adult CNS. J Neurochem 107, 1–19 (2008).
Silvestroff, L. et al. Cuprizone-induced demyelination in CNP::GFP transgenic mice. The Journal of comparative neurology 518, 2261–2283 (2010).
Smith, J.P., Hicks, P.S., Ortiz, L.R., Martinez, M.J. & Mandler, R.N. Quantitative measurement of muscle strength in the mouse. J Neurosci Methods 62, 15–19 (1995).
Moore, C.S., Abdullah, S.L., Brown, A., Arulpragasam, A. & Crocker, S.J. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research 89, 13–21 (2011).
Ishibashi, T. et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 (2006).
Piaton, G., Gould, R.M. & Lubetzki, C. Axon-oligodendrocyte interactions during developmental myelination, demyelination and repair. Journal of neurochemistry 114, 1243–1260 (2010).
Zhou, Q. & Anderson, D.J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109, 61–73 (2002).
Acknowledgements
This work was supported by grants from the National Institutes of Health (RO1 NS059043 and RO1 ES015988 to W.D.; RO1 NS025044 to D.P), National Multiple Sclerosis Society (to W.D.) and Shriners Hospitals for Children (to W.D.). M.M.S. was supported by a postdoctoral fellowship from the California Institute for Regenerative Medicine (CIRM). We also thank Richard Lu at the UT Southwestern Medical Center for providing us with the cDNA for mouse Zfp488. We gratefully acknowledge technical support provided by Peter Takeuchi for the behavior tests; Fuzheng Guo, Erica McCauley and John Avery for immunohistochemistry.
Author information
Authors and Affiliations
Contributions
M.M.S., V.S., U.L., M.S.G. and D.H.F performed the experiments. M.M.S., V.S., D.E.P. and W.D. designed the experiments. W.D. directed the study. All authors participated in writing the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Soundarapandian, M., Selvaraj, V., Lo, UG. et al. Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci Rep 1, 2 (2011). https://doi.org/10.1038/srep00002
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00002
This article is cited by
-
OLIG2 regulates lncRNAs and its own expression during oligodendrocyte lineage formation
BMC Biology (2021)
-
Development of glial restricted human neural stem cells for oligodendrocyte differentiation in vitro and in vivo
Scientific Reports (2019)
-
Transcriptional profiling and biomarker identification reveal tissue specific effects of expanded ataxin-3 in a spinocerebellar ataxia type 3 mouse model
Molecular Neurodegeneration (2018)
-
Sex differences in microRNA-mRNA networks: examination of novel epigenetic programming mechanisms in the sexually dimorphic neonatal hypothalamus
Biology of Sex Differences (2017)
-
Cuprizone-Containing Pellets Are Less Potent to Induce Consistent Demyelination in the Corpus Callosum of C57BL/6 Mice
Journal of Molecular Neuroscience (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.