« Prev Next »

Unlike the gene-poor Y chromosome, the X chromosome contains over 1,000 genes that are essential for proper development and cell viability. However, females carry two copies of the X chromosome, resulting in a potentially toxic double dose of X-linked genes. To correct this imbalance, mammalian females have evolved a unique mechanism of dosage compensation distinct from that used by organisms such as flies and worms. In particular, by way of the process called X-chromosome inactivation (XCI), female mammals transcriptionally silence one of their two Xs in a complex and highly coordinated manner (Lyon, 1961). The inactivated X chromosome then condenses into a compact structure called a Barr body, and it is stably maintained in a silent state (Boumil & Lee, 2001).
A prime example of X inactivation is in the coat-color patterning of tortoiseshell or calico cats (Figure 1). In cats, the fur pigmentation gene is X-linked, and depending on which copy of the X chromosome each cell chooses to leave active, either an orange or black coat color results. X inactivation only occurs in cells with multiple X chromosomes, which explains why almost all calico cats are female.
X inactivation exists in two different forms: random and imprinted. Although both forms utilize the same RNAs and silencing enzymes, they differ in terms of their developmental timing and mechanism of action.
Noncoding RNAs and X Inactivation
As previously mentioned, RNA plays an important role in X inactivation. Specifically, two noncoding, complementary RNAs—XIST and TSIX—initiate and control the inactivation process.
XIST Exists to Silence
XIST, or X-inactive specific transcript, was discovered due to its specific expression from inactive female X chromosomes. This RNA has four unique properties (Borsani et al., 1991; Brockdorff et al., 1991, 1992; Brown et al., 1991, 1992; Clemson et al., 1996):
- The XIST gene does not encode a protein but rather produces a 17 kilobase (kb) functional RNA molecule. Hence, it is a noncoding RNA (Costa, 2008).
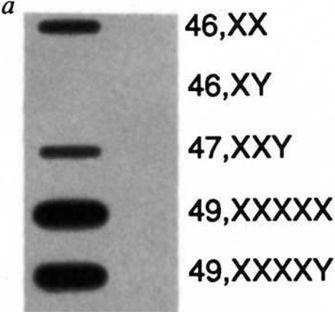
- XIST RNA is only expressed in cells containing at least two Xs and is not normally expressed in male cells (Figure 2). Higher XIST expression can be seen in cells with more X chromosomes, as a counting mechanism dictates that only one X per cell can remain active. In such cells, XIST is expressed from all supernumerary Xs.
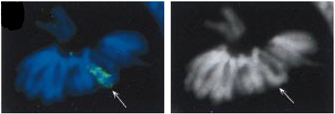
- XIST RNA remains exclusively in the nucleus and is able to "coat" the chromosome from which it was produced (Figure 3).
- Paradoxically, XIST RNA is expressed from an otherwise inactive X chromosome.
Research has shown that XIST RNA is both necessary and sufficient for inactivation (Penny et al., 1996; Wutz & Jaenisch, 2000), and it recruits various silencing protein complexes to label the future inactive X chromosome. Increased XIST expression represents a key initiation event in X inactivation, indicating the central role of this noncoding RNA.
TSIX Antagonizes XIST
Imprinted X Inactivation: Preferential Silencing of the Father
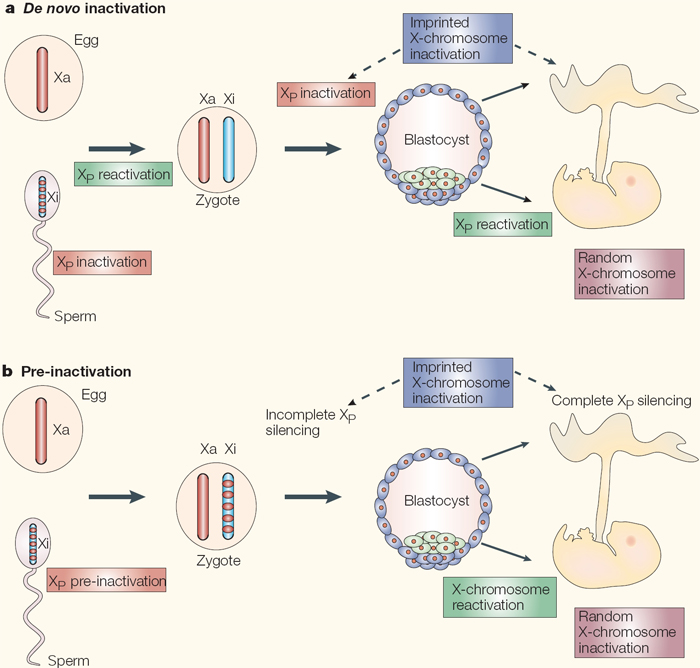
As mentioned earlier in the article, there are two types of X inactivation: imprinted and random. During imprinted X inactivation, the paternal (father's) X chromosome is preferentially silenced in the placenta (an extraembryonic tissue) of eutherian mammals, as well as in all cells of earlier marsupial mammals, such as opossums and kangaroos (Graves, 2006; Huynh & Lee, 2005). Interestingly, XIST and TSIX are both important for imprinted XCI in the mouse (a eutherian), but marsupials do not have an XIST gene homologue (Duret et al., 2006; Lee, 2000; Marahrens et al., 1997; Sado et al., 2001). This raises the question of how imprinted inactivation is achieved in marsupials without using XIST. One possible mechanism involves pre-silencing of the paternal X in the male germ line during a process whereby both sex chromosomes are inactivated during meiosis; here, the inactive state of the X could be transmitted to the next generation (Hornecker et al., 2007; Huynh & Lee, 2005; Namekawa et al., 2007). From an evolutionary point of view, such a form of inherited XCI would have easily and economically achieved dosage compensation in female embryos, because only females inherit an X chromosome from their fathers.
Interestingly, remnants of this type of mechanism may still exist in eutherian mammals, such as the mouse (Huynh & Lee, 2005). While debate remains concerning the details of how and when dosage compensation is achieved, it is clear that imprinted XCI first occurs in all cells of early eutherian mammalian embryos prior to implantation into the uterus (Huynh & Lee, 2005; Okamoto & Heard, 2006). Together with the fact that earlier mammals, such as marsupials, also only silence their paternal Xs, imprinted XCI may represent an evolutionarily ancestral form of X inactivation. Occurring before the appearance of XIST and TSIX, imprinted XCI led to the evolution of random XCI in eutherian mammals.
Random X Inactivation
Random X inactivation occurs in the early female embryo, where both the maternal and the paternal X chromosome have an equal chance of becoming inactivated (Figure 4). Each female cell has the difficult task of trying to distinguish between two X chromosomes within the same nucleus, then designating one as an active X chromosome and the other as an inactive X. This complex process of silencing is accomplished independently in each cell, largely by XIST and TSIX.
Embryonic stem (ES) cells can undergo random X inactivation when differentiated in vitro (Martin et al., 1978; Rastan & Robertson, 1985), and they thus serve as a good model system with which to study this phenomenon. In fact, the use of ES cells along with early mouse embryos has enabled geneticists to dissect the different phases of the random XCI pathway. It seems that each cell first counts its number of X chromosomes, then randomly chooses one X to remain active, and, finally, silences the future inactive X (Bourmil & Lee, 2001). Whole-chromosome silencing involves the recruitment of many specialized factors, such as histone variants and chromatin modifiers (Lucchesi et al., 2005).
In addition to silencing one of the two Xs, the cell must also make sure that the other X remains active. Thus, there must be a way for the two Xs to communicate with each other to designate mutually exclusive fates. Interestingly, recent evidence suggests that this communication is mediated by protein- and transcription-dependent pairing between the Xs during early development (Bacher et al., 2006; Xu et al., 2006, 2007). The random XCI story becomes even more complex with the discovery of various enhancers and modifiers that can alter or skew inactivation of one X chromosome over the other.
Why Evolve Random X Inactivation?
This comparison of imprinted versus random X inactivation raises an important question: If all cells of the early preimplantation embryo already inactivate the paternal X, why is there a need to reactivate it, only to randomly inactivate an X chromosome again? One hypothesis is that perhaps random X inactivation evolved in placental mammals in order to have an additional chance to cope with X-linked mutations. Specifically, if the paternal X is always inactivated, this places the burden of all X-chromosome gene expression on the mother. Placental mammals may have therefore evolved random X inactivation to alleviate the burden of maternal X-chromosome mutations. In Rett's syndrome, for instance, females that carry a mutated copy of the MECP2 gene on the maternal X are able to survive (although with variable symptoms), because the paternal X has a normal copy that remains active in some cells (Ham et al., 2005). Random XCI is also mechanistically more complete than imprinted XCI, which tends to be very leaky, or still exhibit some normal phenotype, in marsupials (Graves, 2006).
Although random XCI is remarkably complete, a significant minority of eutherian genes can escape inactivation and remain active, particularly in humans, despite the fact that XIST RNA spreads along the entire X chromosome (Carrel & Willard, 2005; Johnston et al., 2008). The fact that certain genes can escape silencing directly impacts certain human conditions in which there is an abnormal number of X chromosomes. For example, XO females (Turner's syndrome), XXX females, and XXY males (Klinefelter's syndrome) all display developmental defects (including infertility and congenital heart problems), even though supernumerary Xs are silenced in XXX and XXY individuals (Figure 2), while the single X of the Turner's syndrome female remains active (Ham et al., 2005). These syndromes may be attributed to the fact that X-linked genes that normally escape XCI are not expressed at the correct dosage. X-Inactivation Research: Future Perspectives
X inactivation has been studied for over half a century, yet several unresolved questions remain. For example, how exactly does the cell correctly count its number of Xs? How is silencing achieved and maintained at the molecular level? Why do noncoding RNAs play such a large role in XCI? How do some crucial genes manage to escape from the pervasive influence of XIST on the inactive X?
The study of X inactivation may also provide insight into cancer biology, as two active Xs have been found in many human breast and ovarian tumors (Liao et al., 2003). Additionally, the X-inactivation process can extend beyond the scope of X-linked genes and be applied to many human disorders involving imprinted genes—genes expressed from only one of two parental chromosomes that are apparently also regulated by noncoding RNAs (Sleutels & Barlow, 2002). Without a doubt, X inactivation represents a great model system with which to study a broad range of developmental and epigenetic processes—those involving stable gene expression without changes to the underlying DNA sequence.
References and Recommended Reading
Bacher, C. P., et al. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nature Cell Biology 8, 293-299 (2006) doi:10.1038/ncb1365 (link to article)
Borsani, G., et al. Characterization of a murine gene expressed from the inactive X chromosome. Nature 351, 325–329 (1991) doi:10.1038/351325a0 (link to article)
Boumil, R. M., & Lee, J. T. Forty years of decoding the silence in X-chromosome inactivation. Human Molecular Genetics 10, 2225–2232 (2001)
Brockdorff, N., et al. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351, 329–331 (1991) doi:10.1038/351329a0 (link to article)
———. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71, 515–526 (1992)
Brown, C. J., et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349, 38–44 (1991) doi:10.1038/349038a0 (link to article)
———. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 71, 527-542 (1992)
Carrel, L., & Willard, H. F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404 (2005) doi:10.1038/nature03479 (link to article)
Clemson, C. M., et al. XIST RNA paints the inactive X chromosome at interphase: Evidence for a novel RNA involved in nuclear/chromosome structure. Journal of Cell Biology 132, 259–275 (1996)
Costa, F. F. Non-coding RNAs, epigenetics, and complexity. Gene 410, 9–17 (2008)
Duret, L., et al. The Xist RNA gene evolved in eutherians by pseudogenization of a protein-coding gene. Science 312, 1653–1655 (2006)
Graves, J. A. Sex chromosome specialization and degeneration in mammals. Cell 124, 901–914 (2006)
Ham, A. L., et al. Does genotype predict phenotype in Rett syndrome? Journal of Child Neurology 20, 768–778 (2005)
Hornecker, J. L., et al. Meiotic sex chromosome inactivation in the marsupial Monodelphis domestica. Genesis 45, 696–708 (2007)
Huynh, K. D., & Lee, J. T. X-chromosome inactivation: a hypothesis linking ontogeny and phylogeny. Nature Reviews Genetics 6, 410–418 (2005) doi:0.1038/nrg1604 (link to article)
Johnston, C. M., et al. Large-scale population study of human cell lines indicates that dosage compensation is virtually complete. PLoS Genetics 4, e9 (2008)
Lee, J. T. Disruption of imprinted X inactivation by parent-of-origin effects at Tsix. Cell 103, 17–27 (2000)
Lee, J. T., & Lu, N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell 99, 47–57 (1999)
Lee, J. T., et al. Tsix, a gene antisense to Xist at the X-inactivation centre. Nature Genetics 21, 400–404 (1999) (link to article)
Liao, D. J., et al. Novel perspective: Focusing on the X chromosome in reproductive cancers. Cancer Investigation 21, 641–658 (2003)
Lucchesi, J. C., et al. Chromatin remodeling in dosage compensation. Annual Review of Genetics 39, 615–651 (2005)
Luikenhuis, S., et al. Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Molecular and Cellular Biology 21, 8512–8520 (2001)
Lyon, M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190, 372–373 (1961) doi:10.1038/190372a0 (link to article)
Marahrens, Y., et al. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes & Development 11, 156–166 (1997)
Martin, G. R., et al. X-chromosome inactivation during differentiation of female teratocarcinoma stem cells in vitro. Nature 271, 329–333 (1978) (link to article)
Namekawa, S. H., et al. Sex chromosome silencing in the marsupial male germ line. Proceedings of the National Academy of Sciences 104, 9730–9735 (2007)
Ogawa, Y., et al. Intersection of the RNA interference and X-inactivation pathways. Science 320, 1336–1341 (2008)
Okamoto, I., & Heard, E. The dynamics of imprinted X inactivation during preimplantation development in mice. Cytogenetic and Genome Research 113, 318–324 (2006)
Penny, G. D., et al. Requirement for Xist in X chromosome inactivation. Nature 379, 131–137 (1996) doi:10.1038/379131a0 (link to article)
Rastan, S., & Robertson, E. X chromosome deletions in embryo-derived (EK) cell lines associated with lack of X chromosome inactivation. Journal of Embryology and Experimental Morphology 90, 379–388 (1985)
Sado, T., et al. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development 128, 1275–1286 (2001)
Sleutels, F., & Barlow, D. P. The origins of genomic imprinting in mammals. Advances in Genetics 46, 11–163 (2002)
Stavropoulos, N., et al. A functional role for Tsix transcription in blocking Xist RNA accumulation but not in X-chromosome choice. Proceedings of the National Academy of Sciences 98, 10232–10237 (2001)
Wutz, A., & Jaenisch, R. A shift from reversible to irreversible X inactivation is triggered during ES cell differentiation. Molecular Cell 5, 695–705 (2000)
Xu, N., et al. Transient homologous chromosome pairing marks the onset of X inactivation. Science 311, 1149–1152 (2006) doi:10.1126/science.1122984
———. Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nature Genetics 39, 1390–1396 (2007) doi:10.1038/ng.2007.5 (link to article).