« Prev Next »
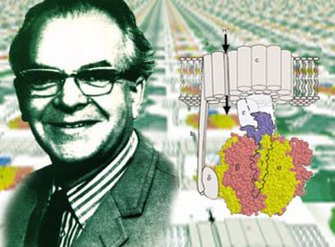
Pioneered by the eccentric British biochemist Peter Mitchell (Figure 1), largely in his own research laboratories in a renovated country house in rural Cornwall, the concept was controversial for more than twenty years. This period of controversy was known as the "ox-phos wars" (after "oxidative phosphorylation," the mechanism of ATP synthesis in respiration). The wars drew to an end only after Mitchell received the Nobel Prize in 1978.
There's an irony here. Mitchell's Nobel was for work in chemistry, yet his ideas are actually about the elimination of chemistry. In the same way that the genetic code enables information to transcend chemistry, so Mitchell's proton gradients enable cellular metabolism to transcend chemistry.
The use of proton gradients gives an insight not only into how life got going in the first place, but also, perhaps, its deepest stalling point: the evolution of complex eukaryotic (nucleated) cells, which arose just once in 4 billion years of evolution.
How Cells Breathe
Our own cells burn food with oxygen and contrive to conserve the energy released in the form of ATP (the universal energy currency of life) — a process called aerobic respiration. How do cells do it? More to the point, how don't they?
Back in the 1940s, Efraim Racker had just figured out the mechanism by which cells glean a little energy from the breakdown of glucose in the absence of oxygen, a pathway known as glycolysis. In glycolysis, phosphate groups are transferred directly from sugar molecules onto ADP to form ATP. The whole pathway is pure chemistry, involving the reaction of one molecule with another, and therefore obeys the laws of stoichiometry; that is, you can balance the equations. Not surprisingly, Racker and others immediately tried to transfer their insights to the quantitatively far more important process of aerobic respiration, which supplies more than 80 percent of our ATP.
But one glaring problem with aerobic respiration is that it doesn't balance. Exactly how much ATP is produced per oxygen molecule consumed? The amount varies, but it's somewhere around 2.5 ATP molecules. That works out to 28–38 ATPs per glucose — again, a variable number, and never an integer (Silverstein 2005). Aerobic respiration is not stoichiometric, so it's really not chemistry. And that's why the long search for a high-energy chemical intermediate (a molecule able to transfer the energy from the oxidation of glucose to form ATP) was doomed to failure.
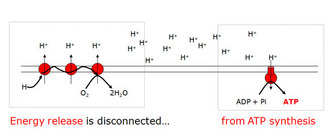
In place of such an intermediate, Mitchell proposed a proton gradient across a membrane: the proton motive force (Mitchell 1961). It works much like a hydroelectric dam. The energy released by the oxidation of food (via a series of steps) is used to pump protons across a membrane — the dam — creating, in effect, a proton reservoir on one side of the membrane. The flow of protons through amazing protein turbines embedded in this membrane powers the synthesis of ATP in much the same way that the flow of water through mechanized turbines generates electricity. This explains why respiration is not stoichiometric: a gradient, by its very nature, is composed of gradations.
Fine Details

The molecular biological achievements of the last two decades culminated in 2010 with the deciphering of the crystal structure of another respiratory complex, the enormous (for a protein) complex I, by Walker's Cambridge colleague Leonid Sazanov (Efremov et al. 2010). Again, the structure betrays the mechanism — in this case not a rotary motor but, even more surprisingly, a lever mechanism not unlike the piston of a steam engine (Figure 2).
Yet without in any way decrying these virtuosic accomplishments, the questions that drove Mitchell in the first place remain surprisingly unanswered. We know in nearly atomic detail how respiration works. We know far less about why it works that way.
Proton Motivation
Mitchell worked on mitochondria because he could; they are a tractable experimental model. But he came at the question from the standpoint of bacterial physiology — how do bacteria keep their insides different from the outside? Throughout his life, Mitchell saw the detailed mechanism of respiration in this far broader sense: Membrane proteins can create gradients across a membrane, and these gradients can in turn power work. Proton gradients powering ATP synthesis were just a special case to Mitchell.
What he can hardly have envisaged so clearly was the pervasive role of protons. Although cells can generate sodium, potassium, or calcium gradients, proton gradients rule supreme. Protons power respiration not only in mitochondria, but also in bacteria and archaea (members of another domain of prokaryotes, which look much like bacteria but have very different biochemistry). Proton gradients are equally central to all forms of photosynthesis, as well as to bacterial motility (via the famous flagellar motor, a rotary motor similar to the ATP synthase) and homeostasis (the import and export of many molecules in and out of the cell is coupled directly to the proton gradient). Even fermenters, which don't need proton gradients to generate ATP, maintain the proton motive force, using ATP derived from fermentation to power proton pumping.
In short, Mitchell knew protons were important, but he could hardly have guessed at just how important. But why protons? Because they were there from the very beginning, says Michael Russell, a geochemist at NASA's Jet Propulsion Laboratory in Pasadena.
Proton Gradients at the Origin of Life
But the centerpiece of Russell's conception lies in natural proton gradients. Four billion years ago, alkaline fluids bubbled into what would then have been mildly acidic oceans (CO2 levels were about a thousand times higher than they are today, and CO2 forms carbonic acid in solution, rendering the oceans mildly acidic). Acidity is just a measure of proton concentration, which was about four orders of magnitude (four pH units) higher in the oceans than in vent fluids. That difference gave rise to a natural proton gradient across the vent membranes that had the same polarity (outside positive) and a similar electrochemical potential (about 200 millivolts [mV] across the membrane) as modern cells have.
Russell has long maintained that natural proton gradients played a central role in powering the origin of life. There are, of course, big open questions — not least, how the gradients might have been tapped by the earliest cells, which certainly lacked such sophisticated protein machinery as the ATP synthase. There are a few possible abiotic mechanisms, presently under scrutiny in Russell's lab and elsewhere. But thermodynamic arguments, remarkably, suggest that the only way life could have started at all is if it found a way to tap the proton gradients (Lane et al. 2010).
Why Proton Gradients Are Necessary
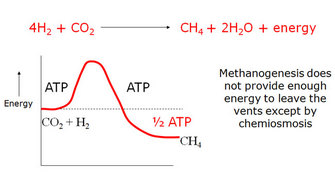
But there's a problem, pointed out by William Martin in collaboration with Russell (Martin & Russell 2007). All cells that use the acetyl-CoA pathway today depend on proton gradients. None of them can grow by fermentation — that is, by the chemistry of glycolysis. Why not? Because CO2 is a stable molecule and does not react easily, even with hydrogen — even when thermodynamics says it should react. CO2 is a bit like oxygen in this respect: Once it starts to react, it's not easily stopped. But a fire needs a spark to get it going, and so, too, does CO2. Cells need the equivalent of a spark to get CO2 to react, and for cells, that spark is ATP. The problem is that the reaction of CO2 with H2 releases energy, but not a lot — only enough to make 1 ATP. That means cells have to spend 1 ATP to gain 1 ATP, so there's no net gain. If there's no gain, there's no growth; no growth, no life.
The Origin of Complex Life
One possible answer relates to the control of proton gradients. All eukaryotic cells turn out to have mitochondria, or once had them and later lost them by reductive evolution back toward a prokaryotic state. No mitochondria, no eukaryotes (Figure 6). All mitochondria capable of oxidative phosphorylation have retained a tiny genome of their own, which appears to be necessary to maintain control over membrane potential (Allen 2003). A membrane potential of 150 mV across the 5-nanometer membrane gives a field strength of 30 million volts per meter — equivalent to a bolt of lightning. This huge electrochemical potential makes the mitochondrial membranes totally different from any other membrane system in the cell (such as the endoplasmic reticulum) which, according to Allen, is why mitochondrial genes are needed locally in cellular subregions. In effect, by responding to local changes in electrochemical potential, they prevent the cell from electrocuting itself. No mitochondrial genome, no oxidative phosphorylation. It could be, then, that bacteria can't expand in cell and genome size because they can't physically associate the right set of genes with their energetic membranes. Lacking mitochondria, bacteria cannot grow large and complex, because they can't control respiration over a wide area of energetic membranes (Lane & Martin, in press). If that's the case, the acquisition of mitochondria and the origin of complexity could be one and the same event.
The question is, what kind of a cell acquired mitochondria in the first place? Most large-scale genomic studies suggest that the answer is an archaeon — that is, a prokaryotic cell that is in most respects like a bacterium. That begs the question, how did mitochondria get inside an archaeon? The answer is a mystery but might go some way toward explaining why complex life derives from a single common ancestor, which arose just once in the 4 billion years of life on Earth.
Summary
Peter Mitchell's demonstration that ATP synthesis is powered by proton gradients was one of the most counterintuitive discoveries in biology, and it took a long time to be accepted. The precise mechanisms by which a proton gradient is formed and coupled to ATP synthesis (chemiosmotic coupling) is now known in atomic detail, but the broader question that drove Mitchell — why are proton gradients so central to life? — is still little explored. Recent research suggests that proton gradients are strictly necessary to the origin of life and highlights the geological setting in which natural gradients form across membranes, in much the same way as they do in cells. But the dependence of life on proton gradients might also have prevented the evolution of life beyond the prokaryotic level of complexity, until the unique chimeric origin of the eukaryotic cell overcame this obstacle.
References and Recommended Reading
Allen, J. F. The function of genomes in bioenergetic organelles. Philosophical Transactions of the Royal Society of London B: Biological Sciences 358, 19–37 (2003)
Efremov, R. G., Baradaran, R. & Sazanov, L. The architecture of respiratory complex I. Nature 465, 441–445 (2010) doi:10.1038/nature09066
Lane, N., Allen, J. F. & Martin, W. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32, 271–280 (2010) doi:10.1002/bies.200900131
Lane, N. & Martin, W. The energetics of genome complexity. Nature 467, 929-934 (2010)
Martin, W. & Russell, M. On the origin of biochemistry at an alkaline hydrothermal vent. Philosophical Transactions of the Royal Society of London B: Biological Sciences 362, 1887–1925 (2007)
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 (1961) doi:10.1038/191144a0
Orgel, L. Are you serious, Dr Mitchell? Nature 402, 17 (1999) doi:10.1038/46903
Russell, M. J. First life. American Scientist 94, 32–39 (2006)
Silverstein, T. The mitochondrial phosphate-to-oxygen ratio is not an integer. Biochemistry and Molecular Biology Education 33, 416–417 (2005)


 Figure 2: The structure of complex I, the largest protein complex involved in respiration in bacteria and mitochondria, as revealed by X-ray crystallography
Figure 2: The structure of complex I, the largest protein complex involved in respiration in bacteria and mitochondria, as revealed by X-ray crystallography