« Prev Next »
Organelles, called plastids, are the main sites of photosynthesis in eukaryotic cells. Chloroplasts, as well as any other pigment containing cytoplasmic organelles that enables the harvesting and conversion of light and carbon dioxide into food and energy, are plastids. Found mainly in eukaryotic cells, plastids can be grouped into two distinctive types depending on their membrane structure: primary plastids and secondary plastids. Primary plastids are found in most algae and plants, and secondary, more-complex plastids are typically found in plankton, such as diatoms and dinoflagellates. Exploring the origin of plastids is an exciting field of research because it enhances our understanding of the basis of photosynthesis in green plants, our primary food source on planet Earth.
Primary Plastids and Endosymbiosis
Where did plastids originate? Their origin is explained by endosymbiosis, the act of a unicellular heterotrophic protist engulfing a free-living photosynthetic cyanobacterium and retaining it, instead of digesting it in the food vacuole (Margulis 1970; McFadden 2001; Kutschera & Niklas 2005). The captured cell (the endosymbiont) was then reduced to a functional organelle bound by two membranes, and was transmitted vertically to subsequent generations. This unlikely set of events established the ancestral lineages of the eukaryote supergroup "Plantae" (Cavalier-Smith 1998; Rodriguez-Expeleta et al. 2005; Weber, Linka, & Bhattacharya 2006), which includes many photosynthetic algae and land plants.
The idea of endosymbiosis was first proposed by Konstantin Mereschkowski, a prominent Russian biologist, in 1905. He coined the term "symbiogenesis" when he observed the symbiotic relationship between fungi and algae (Mereschkowski 1905). The term "endosymbiosis" has a Greek origin (endo, meaning "within"; syn, meaning "with"; and biosis, meaning "living"), and it refers to the phenomenon of an organism living within another organism. In 1923, American biologist Ivan Wallin expanded on this theory when he explained the origin of mitochondria in eukaryotes (Wallin 1923). However, not until the 1960s did Lynn Margulis, as a young faculty member at Boston University, substantiate the endosymbiotic hypothesis. Based on cytological, biochemical, and paleontological evidence, she proposed that endosymbiosis was the means by which mitochondria and plastids originated in eukaryotes (Sagan 1967; Margulis 1970). In those days, the research community viewed her unconventional idea with much skepticism, but her work was eventually published in 1967 (Sagan 1967) after being rejected by fifteen scientific journals! Today, endosymbiosis is a widely accepted hypothesis to explain the origin of intracellular organelles.
Besides these original and bold ideas, what else have we learned? Since 1990 we have seen rapid advancement in techniques in molecular biology and bioinformatics. Using molecular phylogenetic approaches, numerous comparative studies have demonstrated the cyanobacterial origin of genes encoded in the Plantae plastid and provide evidence for gene transfer from the endosymbiont genome to the "host" nucleus (Bhattacharya & Medlin 1995; Delwiche 1999; Moreira, Le Guyader, & Phillippe 2000; McFadden 2001; Palmer 2003; Bhattacharya, Yoon, & Hackett 2004; Rodriguez-Ezpeleta et al. 2005; Reyes-Prieto, Weber, & Bhattacharya 2007). These studies complement several independent lines of evidence based on protein transport and the biochemistry of plastids (McFadden 2001; Matsuzaki 2004; Weber, Linka, & Bhattacharya 2006; Reyes-Prieto & Bhattacharya 2007). The establishment of primary plastids in eukaryotes is estimated to have occurred 1.5 billion years ago (Hedges 2004; Yoon et al. 2004; Blair, Shah, & Hedges 2005), but dating such an ancient event based on molecular data remains controversial due to the limited support provided by the fossil records (Douzer et al. 2004).
Whereas endosymbiosis involving a cyanobacterium explains the establishment of primary plastids in Plantae, the story is more convoluted in other photosynthetic eukaryotes, which harbor secondary plastids with more complex structures. The plastids found in Paulinella chromatophora (a filose amoeba) are an exception to the rule. These organisms are derived from a far more recent cyanobacterial primary endosymbiosis that occurred about 60 million years ago (Bhattacharya, Helmchen, & Melkonian 1995; Marin, Nowack, & Meklonian 2005; Yoon et al. 2006). This plastid traces its origin to a cyanobacterial donor of the Prochlorococcus-Synechococcus type (Yoon et al. 2006). The closely related Paulinella ovalis, although lacking a plastid, is an active predator of cyanobacteria that are commonly localized within food vacuoles (Johnson, Hargraves, & Sieburth 2005). Therefore, the cyanobacterium-derived plastid in the photosynthetic P. chromatophora provides an independent example of the phagotrophic origin of a primary plastid.
Secondary Plastids and the Current Scientific Debate
What is the chromalveolate hypothesis? This controversial idea proposes that a free-living red algal cell was captured and retained by a nonphotosynthetic heterotrophic protist soon after the evolutionary split of red and green algae (i.e., secondary endosymbiosis), giving rise to the pigmented ancestor of the supergroup "Chromalveolata" (Cavalier-Smith 1999). The red algal endosymbiont was retained in a variety of chromalveolate lineages such as cryptophytes, haptophytes, stramenopiles, and dinoflagellates. This hypothesis is based on numerous phylogenetic studies that support the red algal origin of the plastid in most chromalveolates and of most plastid-localized proteins that are encoded in the nucleus of these taxa (Fast et al. 2001; Harper & Keeling 2003; Bhattacharya, Yoon, & Hackett 2004; Li et al. 2006; Nosenko et al. 2006). Although independent horizontal gene transfer events could explain this observation, the more reasonable explanation is that a secondary endosymbiosis event in red alga involved multiple gene transfers.
What other changes have occurred in organisms containing secondary plastids? With the exception of the cryptophytes, which retain a remnant of the red algal cell (Greenwood 1974), other chromalveolates do not have the nucleus of the red algal endosymbiont, which means that the genes necessary to ensure a fully functional plastid have been transferred to the host nucleus (Douglas & Penny 2001; Douglas et al. 2001). In addition, for a subgroup of dinoflagellates that contain the peridinin pigment, including species that cause "red tide" in the ocean (e.g., Alexandrium), the plastid genomes are highly reduced in size due to substantial gene transfer from the endosymbiont to the host nucleus (Hackett 2004).
To further complicate matters, besides tracing their origin not only to red algae as expected, plastid-targeted proteins in many dinoflagellates traced also include proteins derived from other unicellular eukaryotes such as excavates and other chromalveolates (Ishida & Green 2002; Hackett 2004; Yoon et al. 2005; Nosenko 2006), suggesting additional (tertiary) endosymbioses in their evolutionary histories. The recent discovery of a substantial number of red and green algal-derived genes in diatoms (Moustafa et al. 2009) supports the idea of an additional secondary endosymbiotic event with green alga during the early evolution of chromalveolates.
If the chromalveolate hypothesis is true, then why are not all chromalveolates (ciliates and apicomplexans) photosynthetic? Under the assumption of the chromalveolate hypothesis, the lack of plastids in ciliates is explained by subsequent loss of the captured algal cell or by genes of the endosymbiont that were acquired during serial endosymbiosis (Reyes-Prieto, Moustafa, & Bhattacharya 2008). Interestingly, the parasitic Apicomplexans (e.g., Toxoplasma), although nonphotosynthetic, possess unique organelles called the apicoplast (also known as the nonphotosynthetic "plastids") that share similar features with secondary plastids (Maréchal & Cesbron-Delauw 2001; Ralph et al. 2004). The apicoplasts may have shared common origins with secondary plastids of the closely related dinoflagellates, but subsequently lost their photosynthetic capability, likely due to the transition to obligate parasitism (Funes et al. 2002; Waller & McFadden 2005).
Alternative Explanations for the Origin of Secondary Plastids
Is there an alternative explanation for the origin of secondary plastids? Indeed, the independent acquisition hypothesis suggests that the origin of secondary plastids in different groups of chromalveolates (e.g., haptophytes, cryptophytes, and stramenopiles) results from independent, serial endosymbioses involving unicellular eukaryotes, not necessarily red algae (Archibald 2009; Bodyl, Stiller, & Mackiewicz 2009; Baurain et al. 2010). Experimental evidence for this hypothesis is not as strong as that for the chromalveolate hypothesis, however. In fact, many recent analyses are tailored to disprove the chromalveolate hypothesis using selective data sampling (Baurain et al. 2010).
What is the advantage of the independent acquisition hypothesis? We know that over evolutionary time plastid-lacking chromalveolates are likely to lose the plastid and nuclear-encoded plastid targeted proteins derived from the red algal endosymbiont. In evolutionary biology, we assume that the least complex (most parsimonious) explanation for an observation is more plausible. The independent acquisition hypothesis avoids a convoluted explanation of organelle and gene losses to explain the sporadic plastid distribution observed today in nonphotosynthetic chromalveolates. In the extreme case, some scientists who support this hypothesis claim that the fundamental grouping of the chromalveolates is itself inaccurate. Given the paucity of empirical data, we need to understand this complex chain of plastid acquisition events much better.
What about the origin of plastids in other eukaryotes? There are other photosynthetic eukaryotes that are not members of Plantae or Chromalveolata, such as the photosynthetic excavates (e.g., Euglena) and the chlorarachniophyte amoebae (Rhizaria), but the plastid origins of these organisms are less well-studied. Nonetheless a number of phylogenetic analyses show that these organisms have acquired their plastids in more recent instances of secondary endosymbiosis, during which a green alga was independently captured by their common ancestors (Rogers et al. 2007).
Summary
There is no simple way to explain the gain and loss of plastids in all eukaryotes. The origin of primary plastids via endosymbiosis involving a cyanobacterium is well-established, but the origin of secondary plastids is still controversial. However, the chromalveolate hypothesis (and the secondary endosymbiosis involving a red alga) is the best-supported hypothesis to date based on numerous empirical studies. In addition, subsequent tertiary endosymbioses involving other free-living eukaryotes explain plastid origins in other eukaryote lineages. Additional studies and biochemical validation (where possible) are needed to better test existing hypotheses about the evolutionary origins of plastids in eukaryotes.
References and Recommended Reading
Archibald, J. M. The puzzle of plastid evolution. Current Biology 19, R81–R88 (2009) doi:10.1016/j.cub.2008.11.067.
Baurain, D. et al. Phylogenomic evidence for separate acquisition of plastids in cryptophytes, haptophytes, and stramenopiles. Molecular Biology and Evolution 27, 1698–1709 (2010) doi:10.1093/molbev/msq059.
Bhattacharya, D., Helmchen, T. & Melkonian, M. Molecular evolutionary analyses of nuclear-encoded small-subunit ribosomal RNA identify an independent Rhizopod lineage containing the Euglyphina and the Chlorarachniophyta. Journal of Eukaryotic Microbiology 42, 65–69 (1995) doi:10.1111/j.1550–7408.1995.tb01541.x.
Bhattacharya, D. & Medlin, L. The phylogeny of plastids: A review based on comparisons of small-subunit ribosomal RNA coding regions. Journal of Phycology 31, 489–498 (1995) doi:10.1111/j.1529-8817.1995.tb02542.x.
Bhattacharya, D., Yoon, H. S. & Hackett, J. D. Photosynthetic eukaryotes unite: Endosymbiosis connects the dots. Bioessays 26, 50–60 (2004) doi:10.1002/bies.10376.
Blair, J. E., Shah, P. & Hedges, S. B. Evolutionary sequence analysis of complete eukaryote genomes. BMC Bioinformatics 6, 53 (2005) doi:10.1186/1471-2105-6-53.
Bodyl, A., Stiller, J. W. & Mackiewicz, P. Chromalveolate plastids: Direct descent or multiple endosymbioses? Trends in Ecology and Evolution 24, 119–121 (2009) doi:10.1016/j.tree.2008.11.003.
Cavalier-Smith, T. A revised six-kingdom system of life. Biological Reviews 73, 203–266 (1998) doi:10.1111/j.1469-185X.1998.tb00030.x.
Cavalier-Smith, T. Principles of protein and lipid targeting in secondary symbiogenesis: Euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. Journal of Eukaryotic Microbiology 46, 347–366 (1999) doi:10.1111/j.1550-7408.1999.tb04614.x.
Cavalier-Smith, T. Genomic reduction and evolution of novel genetic membranes and protein-targeting machinery in eukaryote-eukaryote chimaeras (meta-algae). Philosophical Transactions of the Royal Society of London, Series B 358, 109–133 (2003).
Delwiche, C. F. Tracing the thread of plastid diversity through the tapestry of life. American Naturalist 154, S164–S177 (1999) doi:10.1086/303291.
Douglas, S. et al. The highly reduced genome of an enslaved algal nucleus. Nature 410, 1091–1096 (2001) doi:10.1038/35074092.
Douglas, S. E. & Penny, S. L. The plastid genome of the cryptophyte alga, Guillardia theta: Complete sequence and conserved synteny groups confirm its common ancestry with red algae. Journal of Molecular Evolution 48, 236–244 (1999) doi:10.1007/PL00006462.
Douzery, E. J. P. et al. The timing of eukaryotic evolution: Does a relaxed molecular clock reconcile proteins and fossils? Proceedings of the National Academy of Sciences 10, 15386–15391 (2004) doi:10.1073/pnas.0403984101.
Fast, N. M. et al. Nuclear-encoded, plastid-targeted genes suggest a single common origin for apicomplexan and dinoflagellate plastids. Molecular Biology and Evolution 18, 418–426 (2001).
Funes, S. et al. A green algal apicoplast ancestor. Science 298, 2155 (2002) doi:10.1126/science.1076003.
Greenwood, A. D. The Cryptophyta in relation to phylogeny and photosynthesis. In Electron Microscopy, eds. Sanders, J. V. & Goodchild, D. J. (Canberra: Australian Academy of Science, 1974): 566–567.
Hackett, J. D. et al. Dinoflagellates: A remarkable evolutionary experiment. American Journal of Botany 91, 1523–1534 (2004) doi:10.3732/ajb.91.10.1523.
Harper, J. T. & Keeling, P. J. Nucleus-encoded, plastid-targeted glyceraldehyde-3-phosphate dehydrogenase (GAPDH) indicates a single origin for chromalveolate plastids. Molecular Biology and Evolution 20, 1730–1735 (2003) doi:10.1093/molbev/msg195.
Hedges, S. B. et al. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evolutionary Biology 4, 2 (2004) doi:10.1186/1471-2148-4-2.
Ishida, K. & Green, B. R. Second-and third-hand chloroplasts in dinoflagellates: Phylogeny of oxygen-evolving enhancer 1 (PsbO) protein reveals replacement of a nuclear-encoded plastid gene by that of a haptophyte tertiary endosymbiont. Proceedings of the National Academy of Sciences 9, 9294–9299 (2002) doi:10.1073/pnas.142091799.
Johnson, P. W., Hargraves, P. E. & Sieburth, J. M. Ultrastructure and ecology of Calycomonas ovalis Wulff, 1919, (Chrysophyceae) and its redescription as a testate Rhizopod, Paulinella ovalis N. Comb. (Filosea: Euglyphina). Journal of Protozoology 35, 618–626 (1988) doi:10.1111/j.1550-7408.1988.tb04160.x.
Keeling, P. J. Chromalveolates and the evolution of plastids by secondary endosymbiosis. Journal of Eukaryotic Microbiology 56, 1–8 (2009) doi:10.1111/j.1550-7408.2008.00371.x.
Kutschera, U., & Niklas, K. J. Endosymbiosis, cell evolution, and speciation. Theory in Biosciences 124, 1–24 (2005) doi:10.1016/j.thbio.2005.04.001.
Li, S. et al. Phylogenomic analysis identifies red algal genes of endosymbiotic origin in the chromalveolates. Molecular Biology and Evolution 23, 663–674 (2006) doi:10.1093/molbev/msj075.
Maréchal, E. & Cesbron-Delauw, M. F. The apicoplast: A new member of the plastid family. Trends in Plant Science 6, 200–205 (2001) doi:10.1016/S1360-1385(01)01921-5.
Margulis, L. Origin of Eukaryotic Cells. New Haven: Yale University Press, 1970.
Marin, B., Nowack, E. C. M. & Melkonian, M. A plastid in the making: Evidence for a second primary endosymbiosis. Protist 156, 425–432 (2005). doi:10.1016/j.protis.2005.09.001.
Matsuzaki, M. et al. Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428, 653–657 (2004) doi:10.1038/nature02398.
McFadden, G. I. Primary and secondary endosymbiosis and the origin of plastids. Journal of Phycology 37, 951–959 (2001) doi:10.1046/j.1529-8817.2001.01126.x.
Mereschkowski C. Über Natur und Ursprung der Chromatophoren im Pflanzenreiche. Biol. Centralbl. 25, 593–604 (1905).
Moreira, D., Le Guyader, H. & Philippe, H. The origin of red algae and the evolution of chloroplasts. Nature 405, 69–72 (2000) doi:10.1038/35011054.
Moustafa, A. et al. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science 324, 1724–1726 (2009) doi:10.1126/science.1172983.
Nosenko, T. et al. Chimeric plastid proteome in the Florida "red tide" dinoflagellate Karenia brevis. Molecular Biology and Evolution 23, 2026–2038 (2006) doi:10.1093/molbev/msl074.
Palmer, J. D. The symbiotic birth and spread of plastids: How many times and whodunit? Journal of Phycology 39, 4–11 (2003) doi:10.1046/j.1529-8817.2003.02185.x.
Ralph, S. A. et al. Evolutionary pressures on apicoplast transit peptides. Molecular Biology and Evolution 21, 2183–2194 (2004) doi:10.1093/molbev/msh233.
Reyes-Prieto, A. & Bhattacharya, D. Phylogeny of Calvin cycle enzymes supports Plantae monophyly. Molcular Phylogenetics and Evolution 45, 384–391 (2007) doi:10.1016/j.ympev.2007.02.026.
Reyes-Prieto, A., Moustafa, A. & Bhattacharya, D. Multiple genes of apparent algal origin suggest ciliates may once have been photosynthetic. Current Biology 18, 956–962 (2008) doi:10.1016/j.cub.2008.05.042.
Reyes-Prieto, A., Weber, A. P. & Bhattacharya, D. The origin and establishment of the plastid in algae and plants. Annual Review of Genetics 41, 147–168 (2007) doi:10.1146/annurev.genet.41.110306.130134.
Rodríguez-Ezpeleta, N. et al. Monophyly of primary photosynthetic eukaryotes: Green plants, red algae, and glaucophytes. Current Biology 15, 1325–1330 (2005) doi:10.1016/j.cub.2005.06.040.
Rogers, M. B. et al. The complete chloroplast genome of the chlorarachniophyte Bigelowiella natans: Evidence for independent origins of chlorarachniophyte and Euglenid secondary endosymbionts. Molecular Biology and Evolution 24, 54–62 (2007) doi:10.1093/molbev/msl129.
Sagan, L. On the origin of mitosing cells. Journal of Theoretical Biology 14, 225–274, IN1-IN6 (1967) doi:10.1016/0022-5193(67)90079-3.
Waller, R. F. & McFadden, G. I. The apicoplast: A review of the derived plastid of Apicomplexan parasites. Current Issues in Molecular Biology 7, 57–80 (2005).
Wallin, I. E. The mitochondria problem. American Naturalist 57, 255 (1923) doi:10.1086/279919.
Weber, A. P., Linka, M. & Bhattacharya, D. Single, ancient origin of a plastid metabolite translocator family in Plantae from an endomembrane-derived ancestor. Eukaryotic Cell 5, 609–612 (2006) doi:10.1128/EC.5.3.609-612.2006.
Yoon, H. S. et al. A molecular timeline for the origin of photosynthetic eukaryotes. Molecular Biology and Evolution 21, 809–818 (2004) doi:10.1093/molbev/msh075.
Yoon, H. S. et al. Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Molecular Biology and Evolution 22, 1299–1308 (2005) doi:10.1093/molbev/msi118.
Yoon, H. S. et al. Minimal plastid genome evolution in the Paulinella endosymbiont. Current Biology 16, R670–R672 (2006). doi:10.1016/j.cub.2006.08.018.



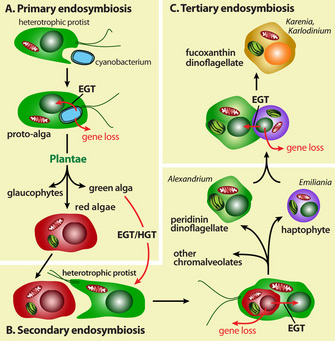
 Figure 1: The concept of endosymbiosis.
Figure 1: The concept of endosymbiosis.


























