« Prev Next »
Signaling between Ephs and ephrins is a major component in determining how neurons wire together during development. This signaling is particularly important in the subfield of axon guidance, where the action of Ephs and ephrins has been heavily studied. The exaggerated cytoskeletal structures of neural cells, which feature extensive dendritic or axonal protrusions that extend many times the length of the cell body, terminate in easily defined structures called "growth cones" while migrating. Due to their large size and accessibility, these dynamic growth cone structures are particularly easy for investigators to study both in vivo and in cell culture systems. Accordingly, examples of the Ephs and ephrins in different aspects of neural development work well to illustrate how they function as guidance molecules during development as well as the range of responses that can come with Eph-ephrin signaling.
Signaling in the Development of the Corticospinal Tract
How does Eph-ephrin signaling influence cell organization during development? A great example comes from the neural wiring of the corticospinal tract (CST), which is a pathway that sends messages from the brain to moving limbs. This pathway depends on the crossing of neurons from one side of the central nervous system (CNS) to the other. Evidence of this anatomical crossing of the CST comes from stroke victims, whose hemorrhage on one side of the brain typically causes weakness or paralysis on the opposite side of the body. The cause is that axons extending from neurons in the motor cortex of one hemisphere of the brain typically connect to targets on the opposite side of the spinal column. Therefore, the left side of the brain controls limb movement on our right side, and the right side of the brain controls limb movement on our left side. During development, axons must grow a great distance — from the motor cortex in the brain to the spinal cord — to complete this connection. When axons of corticospinal neurons from the motor cortex cross the middle of the hindbrain, they plunge down the opposite side of the spinal cord and connect to motor neurons that control movement. In normal development, a metaphorical gate closes along the midline of the brain such that after the axons cross, they cannot go back to the side they came from. This gate closing is a fundamental point in time in development, and it helps keep the wiring of the CNS organized and specific. What does this crossover of the CST neurons mean for motor function? The ultimate result of proper CST wiring is the ability to move asymmetrically, meaning that the right and left feet and arms can move in opposite directions at the same time.
Eph-ephrin Signaling in the CST Affects Mouse Locomotion
Eph-ephrin Signaling in the Development of Vision Pathways
Although CST development gives a more typical example of how Eph-ephrin signaling is put to use during development, the signaling during the development of the visual system in vertebrates exemplifies the diversity that these molecules can have. Consider the challenges during eye development: All axons from the retina must properly connect to a precise spatial pattern in the brain, carrying the spatial information of the visual field captured by the eye. The process of making the correct connections starts when all retinal ganglion cells (RGCs) that carry this information from the retina to the brain must precisely funnel into the narrow optic disc in the middle of the retina to form the optic nerve. These RGC axons then navigate toward the midline of the brain at the optic chiasm, where some axons cross the midline and some do not. The proper migration of RGC axons thus contains three critical decision points: (1) Within the retina, projecting RGC axons must find and navigate through the optic disc to exit the eye and travel into the optic nerve; (2) at the optic chiasm, where the optic nerves from the two eyes connect, RGC axons must either continue migrating across the midline to their intended target in the brain or stay on the same side and travel to same-side targets in the brain; and (3) RGC axons must project directly from the retina toward other areas of the brain, such as the superior colliculus. The process of RGC pathfinding toward the superior colliculus is extremely well orchestrated, with RGC axons originating from specific fields within the retina terminating in specific zones within the superior colliculus in a highly reproducible fashion. As it turns out, researchers have shown that Eph-ephrin signaling is a major player in properly navigating these axons through all three of these RGC pathways.
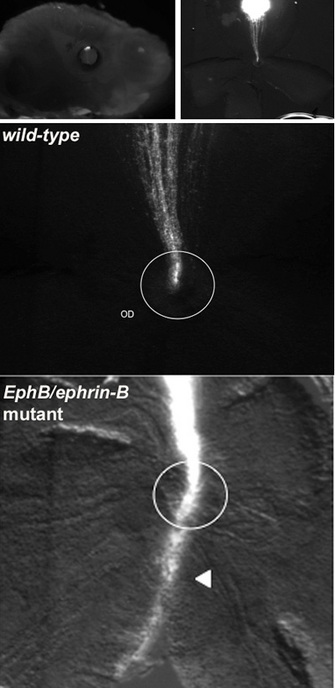
How could these investigators test this hypothesis? One way was to examine a particular kind of mutant mouse wherein forward signaling through the EphB receptor is knocked out. Mark Henkemeyer had created such a mouse strain, called EphB2lacZ. In this mouse, the cytoplasmic domain of EphB2 (containing all the intracellular signaling structures) was deleted and replaced with conjugated b-galactosidase, a reporter gene. If the hypothesis about EphB receptors mediating repulsive cues through reverse signaling was correct about reverse signaling, then mice with the EphB2lacZ mutation should not show any defects in RGC pathfinding to the optic disc because they are only lacking forward signaling function. When the researchers repeated these DiI axon tracing experiments in EphB2lacZ mutant animals, they indeed found that RGC pathfinding was normal. From these observations, they were able to conclude that proper guidance of dorsal RGCs into the optic disc is controlled by EphB activation of reverse signaling through ephrin-B receptors on RGC axons, with a cell-axon repulsive outcome.
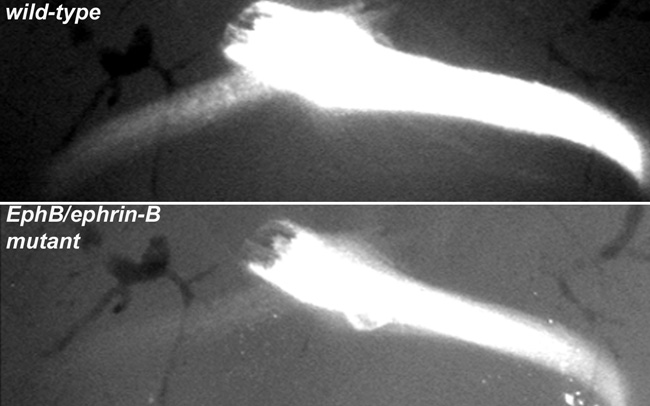
What about RGC axons continuing into the optic chiasm? Like humans, mice have binocular vision, meaning that both eyes perceive the central portion of the visual field. To permit the accurate processing of visual signals, RGCs covering this binocular field within the retinas from both eyes should therefore come together at the same target area in the brain. To ensure that this joining occurs, a subset of RGC axons extending from the ventrotemporal region of the optic disc segregates from the rest of the RGCs at the optic chiasm and instead projects with RGCs from the retina and joins those from the opposite eye. The first suggestion that Eph-ephrin signaling was involved in this process of wiring correctly for binocular vision came from expression studies that compared different animals. These studies showed that ephrin-B2 was expressed in the optic chiasm of mammals (which have binocular vision), but not in the chiasm of fish or chickens (which do not have binocular vision) (Nakagawa et al. 2000), which suggested that ephrin-B2 was involved in pathfinding cues for binocular vision. But how was it involved? In 2003, the role of ephrin-B2 in this pathfinding process was clarified through collaborative research between the laboratories of Carol Mason at the Columbia University Medical Center and Henkemeyer at the University of Texas Southwestern Medical Center. These investigators found that the EphB1 receptor is expressed exclusively in the ventrotemporal region of the retina and that EphB1 null mice show dramatically reduced binocular projections (Williams et al. 2003). What did this finding mean? It indicated to the researchers that ephrin-B2, the ligand for these receptors, both serves as a midline barrier to activate forward signaling in EphB1-positive cells, which repels ventrotemporal RGC axons from the optic chiasm, and correctly directs them to the proper side of the brain to complete the pathway for binocular vision. In the absence of EphB1, these ventrotemporal axons are unable to detect this midline barrier and do not join this critical binocular pathway (Figure 3). This signaling mechanism is different from that in the optic disc, although both do involve repulsion events. Whereas RGC guidance into the optic disc was mediated by repulsive ephrin-B reverse signaling, at the optic chiasm it is EphB forward signaling that provides the critical repulsive instruction.

Future Research for the Ephs and Ephrins in the CNS
Why are scientists so interested in how neural networks are wired? Obviously, axon guidance is a major developmental phenomenon, and knowing how it works means that we may be able to fix defects in axonal connections resulting from injury or disease. For example, spinal cord injuries often result in paralysis because axons are severed. Regrowing axons to the proper targets to heal these spinal cord wounds is theoretically possible, if the proper signals for guiding precise connectivity within the spinal cord were known. In fact, much of the research on Eph-ephrin signaling has potential relevance for developing therapeutics. When function of sensory or locomotion systems does not work properly — due to neurodegeneration in diseases such as diabetes or even due to just normal aging — this dysfunction can severely affect the quality of life. The more scientists are able to understand the molecules involved in guiding these neural structures to their intact forms, the closer we come to better orchestrating some control over repairing these same neural structures when they become damaged.
References and Recommended Reading
Birgbauer, E. et al. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development 127, 1231–1241 (2000).
Dottori, M. et al. EphA4 (Sek1) receptor tyrosine kinase is required for the development of the corticospinal tract. Proceedings of the National Academy of Sciences 95, 13248–13253 (1998).
Hindges, R. et al. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron 35, 475–487 (2002) doi:10.1016/S0896-6273(02)00799-7.
Nakagawa, S. et al. Ephrin-B regulates the ipsilateral routing of retinal axons at the optic chiasm. Neuron 25, 599–610 (2000) doi:10.1016/S0896-6273(00)81063-6.
Williams, S. E. et al. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron 39, 919–935 (2003) doi:10.1016/j.neuron.2003.08.017.
Yokoyama, N. et al. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron 29, 85–97 (2001) doi:10.1016/S0896-6273(01)00182-9.



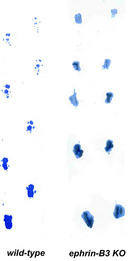
 Figure 1
Figure 1


























