« Prev Next »

RTK
Although all cell membrane receptors receive and transmit signals from the environment, some of these receptors also double as enzymes. In such cases, the binding of a signaling molecule to the membrane receptor activates the receptor's inherent enzymatic activity. Of the various receptors that exhibit this capability, receptor tyrosine kinases (RTKs) make up the largest class. These cell surface receptors bind and respond to growth factors and other locally released proteins that are present at low concentrations. RTKs play important roles in the regulation of cell growth, differentiation, and survival.
When signaling molecules bind to RTKs, they cause neighboring RTKs to associate with each other, forming cross-linked dimers. Cross-linking activates the tyrosine kinase activity in these RTKs through phosphorylation — specifically, each RTK in the dimer phosphorylates multiple tyrosines on the other RTK. This process is called cross-phosphorylation.
What Do RTKs Look Like?
Once cross-phosphorylated, the cytoplasmic tails of RTKs serve as docking platforms for various intracellular proteins involved in signal transduction. These proteins have a particular domain — called SH2 — that binds to phosphorylated tyrosines in the cytoplasmic RTK receptor tails. More than one SH2-containing protein can bind at the same time to an activated RTK, allowing simultaneous activation of multiple intracellular signaling pathways. Ultimately, RTK activation brings about changes in gene transcription. Signaling becomes complex as signals travel from the membrane to the nucleus, due to crosstalk between intermediates in various signaling pathways in the cell (Figure 1).

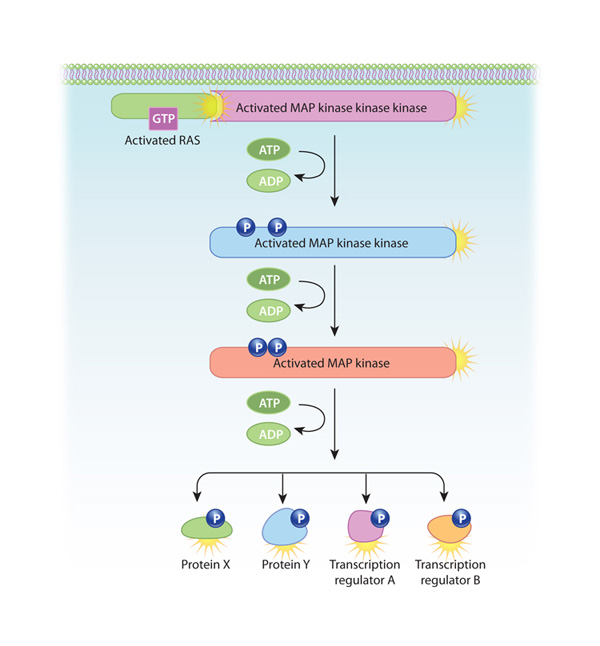
One of the most common intracellular signaling pathways triggered by RTKs is known as the mitogen-activated protein (MAP) kinase cascade, because it involves three serine-threonine kinases. The pathway starts with the activation of Ras, a small G protein anchored to the plasma membrane. In its inactive state, Ras is bound to GDP. However, when SH2-containing proteins join with activated RTKs, they cause Ras to bind GTP in place of GDP and become active. Next, the GTP-bound Ras (which is not itself a kinase) activates the first serine-threonine kinase in the MAP kinase cascade. Each of the three kinases in this cascade then activates the next by phosphorylating it. Because all three kinases in this pathway phosphorylate multiple substrates, the initial signal is amplified at each step. Then, the final enzyme in the pathway phosphorylates transcription regulators, leading to a change in gene transcription (Figure 2). Many growth factors, including nerve growth factor and platelet-derived growth factor, use this pathway.
Not all RTKs use the MAP kinase cascade to send information to the nucleus. For example, insulin-like growth factor receptors activate the enzyme phosphoinositide 3-kinase, which phosphorylates inositol phospholipids in the cell membrane, leading in turn to a protein kinase cascade (distinct from the MAP kinase cascade) that relays the signal to the nucleus. Other RTKs use a more direct route to the nucleus. Transcriptional regulators known as STAT proteins, an acronym for signal transducers and activators of transcription, bind to the phosphorylated tyrosines in the receptors for cytokines and some hormones. Once activated, STAT proteins move directly into the nucleus, causing changes in transcription.
How Do RTK Signals Regulate Cells?
Cells possess many different RTKs that bind to a diverse set of extracellular signaling molecules, many of which are locally produced and present in low concentrations. These local cell-to-cell interactions are important for developing and maintaining the spatial orientation of tissues, which is crucial for higher-level functioning.
Growth factors and hormones are two especially important categories of signaling molecules that bind to RTKs. These molecules direct cell differentiation by determining patterns of gene transcription. Extracellular matrix proteins and certain surface proteins on neighboring cells can also bind to and activate RTKs. For example, upon binding to RTKs, surface proteins called ephrins help guide developmental processes involved in tissue architecture, final placement of nerve endings, and blood vessel maturation.
When RTKs don't function properly, cell growth and differentiation go awry. For instance, many cancers appear to involve mutations in RTKs. For this reason, RTKs are the targets of various drugs used in cancer chemotherapy. For example, the breast cancer drug Herceptin is an antibody that binds to and inhibits ErbB-2 — an RTK that is overexpressed in many metastatic breast cancers.
Conclusion
eBooks
This page appears in the following eBook





















