« Prev Next »
Genetic testing and counseling services have been around since about the mid-1970s. Until recently, most of them revolved around reproduction, with most clients being future parents wanting to know whether a fetus (or embryo) might be carrying a potentially fatal mutation, such as the recessive X-linked mutation that causes Duchenne muscular dystrophy (DMD). By undergoing prenatal testing, parents that suspect their fetus might be carrying the mutation (due to family history or other reasons) can plan accordingly, and either terminate the pregnancy or prepare for a disabled child, depending on their beliefs. Until recently, when people spoke of "genetic testing," they nearly always meant this type of prenatal screening.
While prenatal screening remains a significant portion of the genetic testing and counseling industry, the industry is changing. Over the past several years, with scientists uncovering mutation-disease associations at a remarkably rapid pace, a growing number of commercial tests have been developed that allow individuals to obtain information about their likely susceptibility to developing certain diseases based on the presence (or absence) of one or more mutations associated with those diseases. In one study, 60% of primary care physicians surveyed across the United States reported having ordered genetic tests for patients, and 74% reported having referred patients to a genetic testing or counseling center (Shields et al., 2008).
Widespread use of genetic testing is not without controversy, however. Public health and social science experts are concerned that many genetic tests are of "questionable prognostic value" for two reasons (Wang et al., 2004). First, many of these tests are for medical conditions for which there are few or no known effective risk-reduction strategies (e.g., Alzheimer's, many cancers). In other words, what's the point of being tested without a clear course of action when testing positive? In such situations, testing positive could create more distress than not knowing your genetic susceptibility. Second, even when there are preventative steps that can be taken after testing positive for a disease-associated mutation, the most that many tests reveal is whether or not you are at an increased risk for the disease in question (compared to individuals who test negative), not whether you will actually develop the disease (or when). This is because most common human health disorders, such as cardiovascular disease and most cancers, are caused by a complex interplay of multiple factors. Even for many of the best-studied gene-disease associations, genetic testing cannot determine with certainty whether and when the disease will develop.
BRCA1 and BRCA2 Testing
Such uncertainty can be seen in BRCA1 and BRCA2 testing, for example. Mutations in both genes have been associated with increased risk for breast and ovarian cancers. (Data on ovarian cancer risk associated with altered BRCA genes are not as thorough as they are for breast cancer; therefore, they will not be considered here.) According to the American Cancer Society, estimates of the percentage of BRCA-positive women who will develop breast cancer sometime in the future range from 36% to 85% (i.e., 360 to 850 women out of every 1,000 who test positive). In comparison, scientists estimate that in the general population, about 13.2% of women (132 out of each 1,000) will develop breast cancer. These figures mean that women with mutated BRCA1 or BRCA2 genes are three to seven times more likely to develop breast cancer than women without altered genes.
So, what exactly does a positive BRCA1 or BRCA2 test mean? At best, it tells individuals that their risk of developing breast cancer—the chance that they could become ill—is between 36% and 85%. Positive test results do not provide any indication whatsoever as to whether an individual will actually develop cancer (or when). After all, the actual onset of breast cancer depends on many factors, including family history. Fortunately for those people who do test positive for BRCA1 and BRCA2 mutations, researchers have done enough genomic epidemiological work to home in on the especially high-risk individuals, the ones at the higher end of that 36%-85% risk range. These include individuals from families with multiple cases of breast cancer; individuals from families that have experienced cases of both breast and ovarian cancer; individuals with one or more family members that have each had two original tumors at different sites (e.g., both colon cancer and lymphoma); and people of Ashkenazi (Eastern European) Jewish descent.
In the case of BRCA1 and BRCA2, women who test positive have several follow-up options, but it is not at all clear which of these risk-reduction strategies is the most effective and under what circumstances. In fact, in some cases, it is unclear whether the strategies are effective at all in women with altered BRCA1 or BRCA2 genes (i.e., while these strategies have been shown to reduce risk among women in the general population, it is not clear whey they reduce risk among women who test positive for BRCA). Such strategies include the following:
- Increased surveillance (i.e., monitoring for symptoms more often so that if cancer does develop, it is detected at the earliest possible stage)
- Risk-reduction surgery (i.e., surgical removal of healthy breast or ovarian tissue, although some women have developed cancer even after having at-risk tissue removed)
- Risk avoidance (i.e., changing behavior and lifestyle, by exercising regularly and limiting alcohol consumption, for example, in order to decrease risks that have been associated with cancer in the general population)
- Chemoprevention (e.g., the use of tamoxifen, which has been shown to reduce the risk of invasive breast cancer in women in the general population by as much as 49%)
The fact that test results are not definitive (and cannot possibly be definitive, given the complex genetic and environmental etiology of breast cancer), combined with the reality that nobody really knows which risk-reduction strategy is most effective, makes for very difficult decision making among individuals who test positive. For example, in a British Medical Journal essay on the ethical issues of risk-reduction mastectomy, oncology specialist Francois Eisinger told this story:
"At the end of the consultation, I was summarizing the situation and acknowledging the likelihood that the woman (who was disease-free) belonged to a high-risk family; my advice, at that time, was to dismiss the ‘standard' screening procedure: a mammography every other year starting at the age of 50; and adopt a ‘personalized' one: a mammography every year starting at 30. After a moment of silence, the women very quietly, but sadly, just answered: ‘Yes ... that's what my sister did ... she is dead.'"
Of course, the concern expressed by WHO-that many genetic tests are of "questionable prognostic value"-is not directed specifically toward BRCA1 or BRCA2 testing. Ever since the BRCA genes were identified as being associated with breast cancer in the mid-1990s, scientists have been hot on their trail, conducting studies, gathering information, and constantly learning more about the nature of the association. Public health and social scientists have also jumped on board, studying the consequences of BRCA1 or BRCA2 testing and how best to counsel patients undergoing this testing. In other words, researchers have accumulated an impressive body of knowledge around the association among BRCA1 and BRCA2, breast cancer, and risk-reduction options for individuals that test positive. Still, nothing can change the fact that a risk is just a risk, not a guarantee, and follow-up decisions for women who test positive are extremely difficult, even with adequate counseling.
Rather, the WHO statement reflects widespread concern that, as gene-disease association data accumulate and genetic testing technology continues to advance, companies will start offering, and physicians will start ordering, genetic testing for a growing number of gene-disease associations before an extensive knowledge base has been built up around either the associations themselves (e.g., what other factors, besides genetics, matter), or around how best to counsel patients undergoing testing for those particular associations (especially when there are no clear risk-reduction options).
Factor V Leiden Testing
Unlike BRCA1 and BRCA2 testing, factor V Leiden testing is not something you hear much about in the news. Yet, this is the most commonly ordered DNA-based genetic test in the United States. Factor V Leiden is an inherited blood clotting disorder caused by a mutation in the F5 gene, which encodes a protein called coagulation factor V. Individuals with mutated F5 genes have a two- to eightfold increased risk of venous thromboembolism (i.e., blood clots in the veins, also referred to as VTEs), which can be fatal. In the United States alone, an estimated 600,000 people are hospitalized with VTEs every year.
As with BRCA1 and BRCA2, testing positive for the F5 mutation associated with the disorder doesn't mean that a person is actually going to suffer another VTE. (Factor V Leiden tests are usually ordered for patients that have already had VTEs.) In fact, only about 10%-30% of individuals who test positive experience another VTE at some point in the future. Moreover, as opposed to the situation with BRCA testing, the implications of a positive factor V Leiden test are far from clear. In other words, say you test positive. What comes next? Nobody really knows. While there have been a few suggestions for risk reduction, such as the use of anticoagulant prophylaxis (e.g., taking an aspirin every day), scientists have yet to actually study any of these risk-reduction interventions to see whether they actually work. This raises a critical question: What is the point of knowing whether you are positive for the factor V Leiden mutation, given that nobody really knows what to do with the information?
The Future of Genetic Testing
While testing positive for the gene associated with an increased risk of VTE might not be nearly as psychologically devastating as testing positive for breast cancer-associated BRCA1 or BRCA2, and the clinical implications are not nearly as serious, consider the wide range of new genetic tests that will inevitably be developed for many of the gene-disease associations currently being discovered. What would be the point of getting tested for a gene associated with Alzheimer's, for example, particularly if the gene-disease association were not very clear and, worse, if there was nothing you could do to reduce your risk? But just because we do not have all the answers today, should we stop trying to learn more?
Questioning the purpose of genetic testing may throw a wrench into the genetic testing industry, although many would argue that it is a strong call for more regulatory oversight. Few people would deny that genetic testing is only going to continue to expand, with more and more physicians ordering tests or referring their patients to genetic testing and counseling centers for a growing number of conditions. Rather, the question underscores the need for additional scientific research. It is not enough to know that gene X is associated with disease Y. Scientists also need to determine which risk-reduction strategies work best and under what circumstances.
Those individuals pursuing "risk" genetic testing of any sort will gain information. How beneficial or useful this information will be remains unknown. Such information may motivate patients to stay current on research findings relevant to their clinical situation and be prepared with questions. For many, this may be reason enough to pursue testing. Physicians need to be cognizant of patients' motivations and expectations to be able to best counsel them prior to any testing.
References and Recommended Reading
Burke, W., et al. Genetic test evaluation: Information needs of clinicians, policy makers, and the public. American Journal of Epidemiology 156, 311—318 (2002)
Shields, A. E., et al. Differential use of available genetic tests among primary care physicians in the United States: Results of a national survey. Genetics in Medicine (2008)
Wang, C., et al. Assessment of genetic testing and related counseling services: Current research and future directions. Social Science and Medicine 58, 1427—1442 (2004)



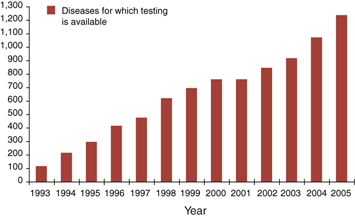
 Figure 1: Growth of genetic testing, including both clinical and research testing.
Figure 1: Growth of genetic testing, including both clinical and research testing.


























