« Prev Next »
No one wants to pass on a debilitating physical condition to the next generation, but all pregnancies carry some degree of risk. Fortunately, it is now possible to detect hundreds of genetic mutations and chromosomal abnormalities very early in the course of human development. Depending on their ages and family histories, prospective parents may be offered a range of diagnostic options to help them understand their child's genetic identity. Early detection of a fetal condition gives parents the opportunity to terminate the pregnancy in an early stage or to make the preparations necessary to best care for an affected child. The decision to undergo prenatal testing should not be made lightly, however, because the testing procedures carry a degree of health risk to both the mother and the unborn child.
Obtaining Fetal DNA
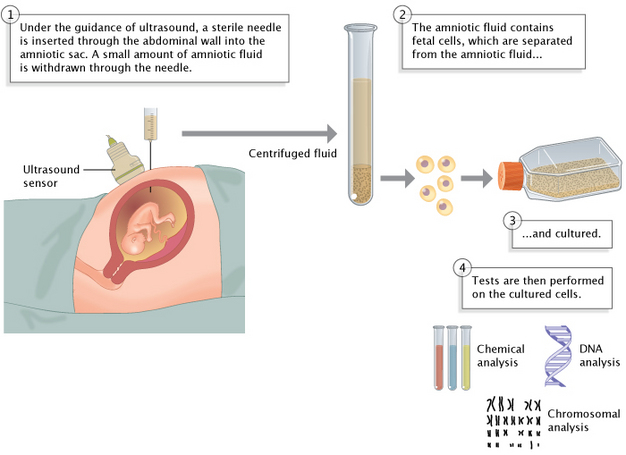
The first step in prenatal testing is obtaining DNA from the unborn child. The oldest procedure for doing this is amniocentesis, which is usually performed between 15 and 18 weeks of pregnancy. Over the years, the safety of amniocentesis has improved significantly, but it still carries a risk of miscarriage or infection that is estimated at about 1 in 400. In amniocentesis (Figure 1), ultrasound is used to guide a long, hollow needle through the woman's abdominal wall and into the amniotic fluid surrounding the fetus. A small amount of amniotic fluid that contains some cells sloughed off from the fetus is then removed via the needle and cultured for one to two weeks before the cells are ready to be used in genetic testing.
There are also several less invasive techniques that focus on looking for embryonic cells in a mother's blood and plasma, although it is often challenging to separate the mother's DNA from that of the fetus. As techniques for cell separation become more efficient and molecular characterization becomes more sensitive, these noninvasive screening methods will probably become more popular, because they offer the prospect of safe and relatively inexpensive cytogenetic analysis.
Testing for Abnormalities in Fetal DNA
Once isolated, fetal DNA can be tested for a wide range of genetic abnormalities, such as maternal-fetal incompatibilities for the rhesus D antigen, as well as other more ethically controversial factors, like the child's sex. Aneuploidies such as Down syndrome can generally be detected by the presence of additional chromosomes or chromosome translocations in a karyotype or fluorescence in situ hybridization (FISH) profile. Healthy individuals may also be unwitting carriers of chromosomal translocations that can be passed down to their children. Translocation carriers have the full complement of genes, but one or more of their chromosomes are abnormal, depending on whether the translocation resulted from chromosome fusion or from a reciprocal exchange between two chromosomes. Carriers of translocations display a range of reproductive problems. Moreover, they sometimes have other children who died in infancy or who have mental retardation, because most of the gametes produced by translocation carriers are aneuploid. Today, the use of genetic testing techniques makes it possible to determine whether a fetus is a healthy translocation carrier.
The availability of a genetic profile for a developing fetus is an important family planning tool. Its only shortcoming is that by the time these tests can be performed, the pregnancy is already well under way, so the parents often face profound ethical dilemmas regarding family planning. An alternative in vitro testing method called preimplantation genetic diagnosis (PGD) does exist, but it carries with it a whole new set of ethical issues.
Preimplantation Genetic Diagnosis
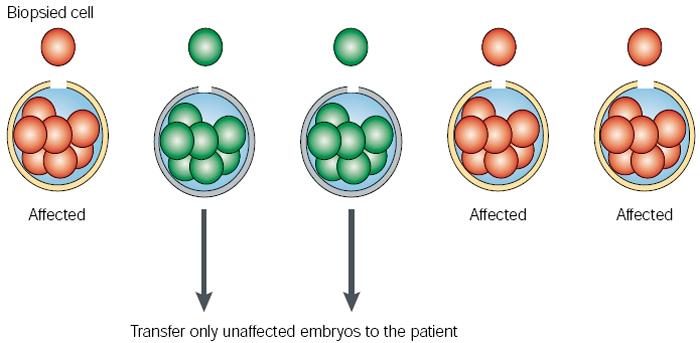
Preimplantation genetic diagnosis now offers prospective parents the option of detecting potential defects in an embryo within the first few days after conception. The idea behind PGD is simple: a single cell, or blastomere, is removed from an embryo that has been fertilized in vitro, and this cell is tested for genetic abnormalities. If the blastomere does not appear to have the defect under investigation, the rest of the embryo can then be transferred to the mother's uterus (Figure 2). Considerable evidence indicates that removal of a single blastomere does not affect the subsequent development of the embryo (Braude et al., 2002).
PGD depends on methods that are routinely used for in vitro fertilization (IVF). Multiple oocytes are first collected from the ovaries of a prospective mother who has been treated with hormones that cause her to "superovulate," and these oocytes are then fertilized using the father's sperm. Successful fertilizations can be detected by the fusion of the maternal and paternal pronuclei, as well as by the extrusion of two polar bodies as the egg completes meiosis. These polar bodies are sometimes collected and analyzed when couples are primarily concerned with the woman's genetic contribution to the embryo, because this procedure provides no information about the father's genetic contribution. More commonly, however, a blastomere is removed from the embryo when it reaches the 8- to 12-cell stage at about three days after fertilization. Once the blastomere is removed for analysis, it is a race against time, because embryos that pass the screening procedure will need to be transferred to the mother's uterus within the next one to two days.
In the first published report of human PGD (Handyside et al., 1990), investigators used Y-chromosome-specific primers in polymerase chain reactions to successfully identify male embryos at risk for X-linked disorders. Since that time, PGD has been used to diagnose many different conditions, and the procedure has become available in many fertility clinics. PGD is most commonly recommended for couples who are at high risk of conceiving a child with serious genetic defects; this includes older mothers at risk for chromosomal abnormalities and couples with high-risk family histories. In addition, recessive gene mutations may not always be apparent in a pedigree, so many couples only become aware that they carry mutant alleles when they have an affected baby. These couples are perfect candidates for PGD for their subsequent pregnancies.
Not surprisingly, error rates in PGD analyses are significantly higher than those in amniocentesis or CVS, which are often recommended as follow-up procedures to PGD. Some critics even argue that PGD "encourages the waste of healthy embryos" (Gosden, 2007) because of the fairly high (but not yet firmly established) rate of misdiagnosis associated with the FISH techniques used to diagnose chromosomal abnormalities in the harvested embryonic cells.
In addition, due to the nature of the embryo-harvesting techniques associated with IVF, ethical dilemmas often arise regarding the fate of excess embryos when PGD is used to select one healthy embryo for implantation. Many couples choose to freeze the surplus embryos in liquid nitrogen; this technique is called cryopreservation. The main factor that determines whether an embryo will survive the deep freeze is the amount of membrane damage it incurs during the process of replacing intracellular liquid with "antifreeze." Embryonic cryopreservation is also a costly option, and there are currently no clear policies in place that determine who bears these costs should the couple later choose to donate their embryos to stem cell research or to another IVF candidate (Heng & Cao, 2006). These issues also bring to light the treatment of embryos as a marketable commodity, especially when their genetic information becomes known.
Thus, prenatal testing techniques are never completely without health risk, but their diagnostic value often outweighs these risks. To ensure these techniques are not misused, any current and future advances in this field must be subject to rigorous ethical regulation.
References and Recommended Reading
Braude, P., et al. Preimplantation genetic diagnosis. Nature Reviews Genetics 3, 941–955 (2002) doi:10.1038/nrg953 (link to article)
Gosden, R. Genetic test may lead to waste of healthy embryos. Nature 446, 372 (2007) doi:10.1038/446372b (link to article)
Handyside, A. H., et al. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature 344, 768–770 (1990) doi:10.1038/344768a (link to article)
Heng, B. C., & Cao, T. Refund fertility-treatment costs for donated embryos. Nature 443, 26 (2006) doi:10.1038/443026a (link to article)
Lo, Y. M. D., & Chiu, R. W. K. Prenatal diagnosis: Progress through plasma nucleic acids. Nature Reviews Genetics 8, 71–77 (2006) doi:10.1038/nrg1982 (link to article).