« Prev Next »
Like animals, plants breathe. The gas exchange into and out of a plant leaf occurs at the underside of leaves, and the process is precisely regulated. What are the gases that are exchanged at the leaf surface? The main energy-producing biochemical process in plants is photosynthesis, a process that, initiated by energy from the sun, converts CO2 and water into carbohydrate energy molecules for the plant and releases O2 back into the atmosphere. In this process, leaves take in atmospheric CO2 and release O2 back into the air. How do plants perform these gas exchange activities between leaf cells and the outside environment? Scientists discovered that a distinct organelle, the vacuole, plays a critical role in regulating the delivery of CO2 to the photosynthesis-conducting chloroplasts.
Plant vacuoles are fluid-filled organelles bound by a single membrane called the tonoplast, and contain a wide range of inorganic ions and molecules. Scientists have identified at least two types of plant vacuoles. The two main types are the protein storage vacuoles of neutral pH, and the lytic vacuoles of acidic pH, which are equivalent in function to lysosomes in mammalian cells (Figure 1).
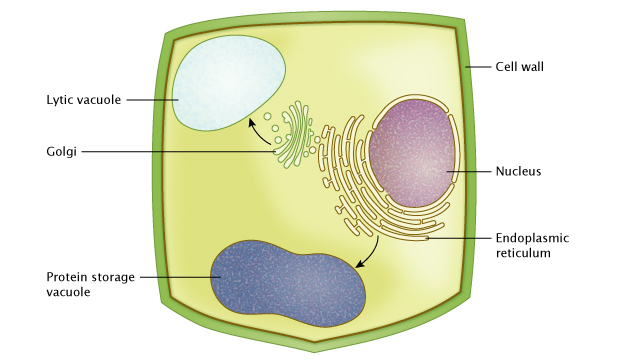
Vacuole Size Changes Are Correlated with Stomatal Movements

How Do Vacuoles Change During Stomatal Opening and Closing?
One way to track dynamic changes in guard cell vacuoles during stomatal movements is to use cell imaging techniques, such as confocal microscopy and TEM. In 2005, Gao et al. did just this when they studied leaf epidermis from the plant Vicia faba using microscopy coupled with fluorescent dyes. First, they removed strips of epidermal cells from leaves, then they stained guard cells with various fluorescent dyes. They used two dyes that specifically attach to vacuoles due to their acidic pH. These dyes cause the vacuoles to glow fluorescent green or red. They also used a green dye that remains in the cytoplasm and does not enter vacuoles. This dye gives an inverse image to the vacuole-specific dyes (Figure 3). With the use of these compartment-specific dyes, they were able to observe the size, shape, and number of vacuoles at various time points during stomatal movements. In their experiment, Gao et al. asked, what happens to the vacuoles and the cytoplasm during stomatal opening and closing? They controlled stomatal action experimentally with known agents. They induced opening with halogen cold-light, and closing with chemical abscisic acid (ABA). During these inductions, they observed that, in the closed state, guard cells contain many small vacuoles, but during stomatal opening, these small vacuoles readily fuse with each other, or with bigger vacuoles. The result is very large vacuoles in guard cells surrounding an open stoma. Conversely, in closing stomata, the large vacuoles once again split into smaller ones, and generate many complex membrane structures. Though these scientists observed a visual coincidence of vacuole changes and stomatal movements, are these dynamic changes necessary for stomatal movements to occur?
Does Stomatal Opening Require Vacuolar Fusion?
To test whether vacuole dynamics are necessary, Gao et al. asked, what would happen to stomatal movements if they experimentally disrupt vacuolar fusion? To investigate this problem, they again turned to their test system, leaf epidermal peels. They treated these peels with a membrane-permeable compound known to inhibit the fusion of endosomes with vacuoles, called E-64d ((2s,3s)-trans-epoxy-succinyl-L-leucylamido-3-methylbutane ethyl ester), and found that the treated guard cells had a greater number of vacuoles than untreated control guard cells. They also observed that stomatal opening was slower in treated guard cells compared to the untreated controls. To explain this, they concluded that interrupted vacuolar fusion has an effect of slowing stomatal opening, and therefore vacuolar fusion must be necessary for stomatal opening to properly function. To explore the genetic basis for vacuolar dynamics, Gao et al. followed up this initial conclusion in Vicia faba with additional experiments using mutant plants. Genetic manipulation in the plant Arabidopsis can produce a mutant that is defective in producing a protein named SGR3. Previous work by other scientists established that SGR3 impacts the transport of vesicles into vacuoles and vacuolar fusion. When Gao et al. compared SGR3 mutants to normal (wild type) plants, they found slower stomatal opening in response to light induction in the mutant plants. With their knowledge of SGR3 function, and these observations, they again concluded that impaired stomatal movement was a consequence of reduced vacuolar fusion in guard cells. Altogether, their results show that fusion of vacuoles is necessary for normal, rapid stomatal movements.
What Are Other Mechanisms for Vacuolar Expansion?
Are there other ways guard cells can increase vacuolar volume aside from fusing small vacuoles? The answer appears to be yes. Until recently, researchers believed that the tonoplast (vacuolar membrane) had a smooth surface. Modern cell imaging techniques with live plant cells have shown otherwise. With confocal microscopy of live cells, several research groups have observed a wavy vacuolar surface, called tonoplast foldings, and vesicle-like structures within the vacuolar lumen (Cutler et al. 2000; Verbelen & Tao 1998; Yamamoto et al. 2003). Using time-lapse imaging, Gao et al. also observed these foldings and vesicle-like structures, which disappeared upon stomatal opening but re-appeared during stomatal closing. This led them to conclude that these observed intravacuolar membrane structures may serve as reservoirs for the tonoplast, so it has membrane ready to supply any needs for rapid expansion. Furthermore, they discovered that what at first appeared to be individual vacuoles might actually be connected ones. They discovered this when they directed a strong beam of excitation light directly onto a single vacuole containing fluorescent dye. This precise beam of light causes photobleaching of dye molecules, and they subsequently observed photobleached dye molecules in both the targeted vacuoles and neighboring ones, indicating that there may be some physical continuity between what appeared to be separate vacuoles. The scientists concluded that neighboring vacuoles are physically linked. Altogether, these experimental observations suggest that vacuoles, tonoplasts, and intravacuolar membrane systems interact to sustain rapid stomatal movements, a prerequisite for CO2 uptake during photosynthesis.
Many questions remain about the exact mechanisms of vacuole expansion that regulate stomatal movements. For instance, why does guard cell volume increase involve expansion of a few big vacuoles instead of the collective expansion of many smaller ones? Fusion of large vacuoles can consume a large amount of energy than the expansion of multiple smaller ones. A possible explanation for this seemingly inefficient energy strategy lies in the tradeoff for volume over surface area. A big vacuole would have a greater total volume/surface area ratio than many small ones. This makes a big vacuole more effective at volume expansion.
Another question is, what is the exact mechanism of vacuolar fusion, and under what conditions does it occur? Many environmental factors affect stomatal movement, and one of them is CO2, the very gas that enters stomata. With the ever-accumulating reports of increasing CO2 concentration in our atmosphere, these questions about stomatal regulation have relevance to issues of global climate change. How will this increasing atmospheric CO2 concentration influence the regulation of guard cell movement? Scientists are currently investigating these phenomena with sophisticated genetic tools and microscopy.
Summary
Plant vacuoles are ubiquitous organelles that are essential to multiple aspects of plant growth, maintenance and development. Their key role in stomatal movements underscores their importance in fundamental gas exchange for plants. Scientists are actively pursuing the exact mechanisms that control vacuole fusion, which supports stomatal movements as well as other plant cell functions. In addition to their role in controlling photosynthetic gas exchange, vacuoles also store compounds that may help to protect photosystems in the chloroplast from damage caused by excess light. Vacuoles are important compartments in plant cell metabolism. An intact vacuole is necessary for many plant functions. Scientists are working toward identifying and characterizing a large and diverse group of tonoplast transporters. They ask: What is their molecular structure? What do they do? How might they be linked? Understanding the answers to these questions is important, as they show how vacuoles are an integrated component of complex cellular networks. As the data above show, vacuoles are crucial to plant cells, as they enable gas exchange mechanisms that optimize metabolic conditions in the cytosol, and allow a plant to react to changing environmental conditions.
References and Recommended Reading
Allen, G.J. et al. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057 (2001)
Brandizzi, F. & Hawes, C. A long and winding road. EMBO reports 5, 245-249 (2004). doi: 10.1038/sj.embor.7400099
Cutler, S.R. et al. Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97, 3718–3723 (2000)
Gao, X. et al. The Dynamic Changes of Tonoplasts in Guard Cells Are Important for Stomatal Movement in Vicia faba. Plant Physiology 139, 1207–1216 (2005)
Gilroy, S. & Trewavas, A. Signal processing and transduction in plant cells: the end of the beginning? Nature Reviews Molecular Cell Biology 2, 307–314 (2001) doi:10.1038/35067109.
Hu, H. et al. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nature Cell Biology 12, 87–93 (2009) doi:10.1038/ncb2009.
Lee, M. et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nature Cell Biology 10, 1217–1223 (2008) doi:10.1038/ncb1782.
Peters, C. & Mayer, A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575–580 (1998)
Sanderfoot, A. A. et al. The t-SNARE AtVAM3p resides on the prevacuolar compartment in Arabidopsis root cells. Plant Physiol 121, 929–938 (1999)
Sato, M et al. The AtVAM3 encodes a syntaxin-related molecule implicated in the vacuolar assembly in Arabidopsis thaliana. J Biol Chem 272, 24530–24535 (1997)
Serna, L. Coming closer to a stoma ion channel. Nature Cell Biology 10, 509–511 (2008) doi:10.1038/ncb0508–509.
Schroeder, J. I. et al. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410, 327–330 (2001)
Surpin, M. & Raikhel, N.. Traffic jams affect plant development and signal transduction. Nature Reviews Molecular Cell Biology 5, 100–109 (2004) doi:10.1038/nrm1311.
Verbelen, J.P. & Tao, W.. Mobile arrays of vacuole ripples are common in plant cells. Plant Cell Rep 17, 917–920 (1998)
Yamamoto, Y. et al. Behavior of vacuoles during microspore and pollen development in Arabidopsis thaliana. Plant Cell Physiol 44, 1192–1201 (2003)






























