« Prev Next »
All our activities — eating, walking, talking — are controlled by our brains, the center of the nervous system. The brain receives huge amounts of information from outside our body via our five senses (vision, sound, taste, touch, and smell), integrates this information, and orders our muscles to take action. How is all that accomplished so efficiently? The answer lies in a membrane structure called myelin.
Information Transmission in the Body
All information both to and from the body must be coordinated and transmitted simultaneously and very quickly. The brain itself requires extremely fast speeds to operate at even at the simplest level. How do the biological tissues of our body support such rapid coordination of the brain, limbs, and sensory input? They do so with nervous system tissue that imitates electrical wiring.
The nervous system is comprised of two primary cell types: neurons and glial cells. These cells communicate with each other to perform important tasks in the nervous system. The glial cells support neurons structurally and maintain their long-term neuronal integrity, and neurons regulate glial cell behavior. In this support of neurons, glial cells have become highly specialized. Glial cells, which can be divided into several types, have various important functions, such as providing structural support, growth support, and insulation around the axon.
Why must glial cells support neurons? Neurons are specialized cells that receive and send signals to other cells through fragile and thin cellular extensions called axons. These axons extend over distances long and short to reach their target, ultimately connecting neurons with other nerve tissue, muscle tissue, or sensory organs (Figure 1A). For example, some motor neurons in the spinal cord have axons that exceed 1 m in length, connecting the spine to the lower limb muscles. These axons transmit signals to the target muscle in the form of electric impulses called action potentials. However, the axons alone are not enough to produce rapid conduction of the electric current necessary for this signal to be sent. Glial cells are the key element for supporting the messages neurons send and receive all over the body. Much like the insulation around the wires in electrical systems, glial cells form a membraneous sheath surrounding axons called myelin, thereby insulating the axon. This myelination, as it is called, can greatly increase the speed of signals transmitted between neurons (known as action potentials). Indeed, the evolution of myelin allowed vertebrates to achieve efficient nervous systems despite their large body size.
The Myelin Sheath: Shape, Size, and Composition
What exactly is myelin? Myelin is a concentrically laminated membrane structure surrounding an axon around which lamellae (or cellular protrusions) repeat radially at a period of about 12 nm (Waxman, Kocsis & Stys 1995; Sherman & Brophy 2005). The myelin lamella is formed by fusion of the apposed inner leaflets of the plasma membrane in glial cells, with no intervening cytoplasm (Figure 1B).
Depending on the location, different glial cell types make myelin in a different manner. Schwann cells make myelin in the peripheral nervous system (PNS: nerves) and oligodendrocytes in the central nervous system (CNS: brain and spinal cord). In the PNS, one Schwann cell forms a single myelin sheath (Figure 1A). By contrast, in the CNS, the oligodendrocyte sends cell processes to myelinate multiple segments on many axons (Figure 2). Although there are several molecular or morphological differences between nerve fibers in the PNS and CNS, the basic myelin sheath arrangement and the electrophysiological characteristics are essentially the same.
Are all axons covered with myelin? No; they can be either myelinated or unmyelinated. Myelinated axons are ensheathed along their entire length. The axon caliber (diameter) in mammalian PNS ranges from 0.1 μm to 20 μm, with unmyelinated axons being less than 2 μm and myelinated axons being more than 1–2 μm in diameter. In the CNS, almost all axons with diameters greater than 0.2 μm are myelinated. In cross section, the myelinated axon appears as a nearly circular profile surrounded by a spirally wound multilamellar sheath (Figure 1C and D). Amazingly, a large myelinated axon may have up to 250 to 300 turns of myelin wrapping around it. The ratio between axon diameter and that of the total nerve fiber (axon and myelin) is 0.6–0.7, a ratio that is well maintained regardless of the axon caliber. The length of the myelin sheath along the axon is approximately 1 mm in the PNS. Between two adjacent myelin segments, there are approximately 1-μm-long gaps called nodes of Ranvier (Figure 1A and E). At the nodes, the axon is exposed to the extracellular space.
Axonal Signaling Regulates Myelination
How is the spiral wrapping of the myelin sheath around axons formed precisely and appropriately? One mechanism has been identified in PNS myelination. In the PNS, neuregulin 1 type III protein is expressed on the axon surface and interacts with glial ErbB receptors, and it has a pivotal role for Schwann cell differentiation and myelination (for review, see Nave & Salzer 2006). Unmyelinated autonomic neurons express low levels of neuregulin 1 type III on the axon surface, whereas heavily myelinated axons express high levels.
Without neuregulin 1 type III, Schwann cells in culture derived from these mutant mice cannot myelinate neurons in the spinal cord (dorsal root ganglion neurons). Intriguingly, in normally unmyelinated fibers, forced expression of neuregulin 1 type III in the postganglionic fibers of sympathetic neurons grown in culture can be forced to myelinate. Thus, the level of neuregulin 1 type III on the PNS axons is a key instructive signal for myelination. Furthermore, above the threshold, the myelin formation is correlated with the amount of neuregulin 1 type III presented by the axon to the Schwann cell. Reduced expression of neuregulin 1 type III leads to a thinner than normal myelin sheath in the heterozygous mutant mice of this molecule. In contrast, transgenic mice that overexpress neuregulin 1 become hypermyelinated.
One interesting question is: Does neuregulin-ErbB signaling regulate CNS myelination as well? Although several reports show that oligodendrocytes respond to neuregulin 1 in vitro, analyses of a series of conditional null mutant animals lacking neuregulin 1 showed normal myelination (Brinkmann et al. 2008). It is still unclear how myelination is regulated in the CNS.
Myelin Promotes Rapid Impulse Transmission Along Axons
How does myelin enhance the speed of action potential propagation? It insulates the axon and assembles specialized molecular structure at the nodes of Ranvier. In unmyelinated axons, the action potential travels continuously along the axons. For example, in unmyelinated C fibers that conduct pain or temperature (0.4–1.2 μm in diameter), conduction velocity along the axon is 0.5–2.0 m/s (as fast as you walk or jog).
In contrast, among the myelinated nerve fibers, axons are mostly covered by myelin sheaths, and transmembrane currents can only occur at the nodes of Ranvier where the axonal membrane is exposed. Myelin is rich in lipids (approximately 80%) and can therefore act as an insulator (meaning high transverse resistance and a low electrical capacitance) along the internodal segments. For example, conduction velocity in the most thoroughly myelinated axons (12–20 μm in diameter) is 70–120 m/s (race car speed), although other factors such as axon caliber can influence this velocity.
At nodes, voltage-gated sodium channels are highly accumulated and are responsible for the generation of action potentials. To induce and maintain nodal sodium channel clusters, specific molecules are also enriched at nodal axons, including cell adhesion molecules such as neurofascin 186 and cytoskeletal and scaffolding proteins such as bIV spectrin (Poliak & Peles 2003; Susuki & Rasband 2008). The myelin helps assemble this nodal molecular organization. For example, during the development of PNS myelinated nerve fibers, a molecule called gliomedin is secreted from myelinating Schwann cells then incorporated into the extracellular matrix surrounding nodes, where it promotes assembly of nodal axonal molecules. Due to the presence of the insulating myelin sheath at internodes and voltage-gated sodium channels at nodes, the action potential in myelinated nerve fibers jumps from one node to the next. This mode of travel by the action potential is called "saltatory conduction" and allows for rapid impulse propagation (Figure 1A).
Myelin Damage Causes Severe Neurological Diseases
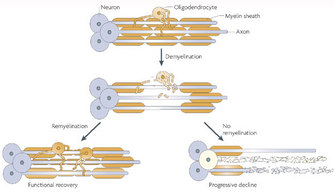
Following demyelination, the naked axon can be re-covered by new myelin. This process is called remyelination and is associated with functional recovery (Franklin and ffrench-Constant 2008). The myelin sheaths generated during remyelination are typically thinner and shorter than those generated during developmental myelination. In some circumstances, however, remyelination fails, leaving axons and even the entire neuron vulnerable to degeneration. Thus, patients with demyelinating diseases suffer from various neurological symptoms.
The representative demyelinating disease, and perhaps the most well known, is multiple sclerosis (MS). This autoimmune neurological disorder is caused by the spreading of demyelinating CNS lesions in the entire brain and over time (Siffrin et al. 2010). Patients with MS develop various symptoms, including visual loss, cognitive dysfunction, motor weakness, and pain. Approximately 80 percent of patients experience relapse and remitting episodes of neurologic deficits in the early phase of the disease (relapse-remitting MS). There are no clinical deteriorations between two episodes. Approximately ten years after disease onset, about one-half of MS patients suffer from progressive neurological deterioration (secondary progressive MS). About 10–15 percent of patients never experience relapsing-remitting episodes; their neurological status deteriorates continuously without any improvement (primary progressive MS). Importantly, the loss of axons and their neurons is a major factor determining long-term disability in patients, although the primary cause of the disease is demyelination. Several immunodulative therapies are in use to prevent new attacks; however, there is no known cure for MS.
Research in Myelin Biology and Pathology
How do we structure a research effort to elucidate the mechanisms involved in developmental myelination and demyelinating diseases? We need to develop useful models to test drugs or to modify molecular expression in glial cells. One strong strategy is to use a culture system. Coculture of dorsal root ganglion neurons and Schwann cells can promote efficient myelin formation in vitro (Figure 1E). Researchers can modify the molecular expression in Schwann cells, neurons, or both by various methods, including drugs, enzymes, and introducing genes, and can observe the consequences in the culture dish.
Modeling demyelinating disease in laboratory animals is commonly accomplished by treatment with toxins injurious to glial cells such as lysolecithin or cuprizone. Autoimmune diseases such as MS or autoimmune neuropathies can be reproduced by sensitizing animals with myelin proteins or lipids (Figure 3). Some mutant animals with defects in myelin proteins and lipids have been discovered or generated, providing useful disease models for hereditary demyelinating disorders. Further research is required to understand myelin biology and pathology in detail and to establish new treatment strategies for demyelinating neurological disorders.
Summary
Myelin can greatly increase the speed of electrical impulses in neurons because it insulates the axon and assembles voltage-gated sodium channel clusters at discrete nodes along its length. Myelin damage causes several neurological diseases, such as multiple sclerosis. Future studies for myelin biology and pathology will provide important clues for establishing new treatments for demyelinating diseases.
References and Recommended Reading
Brinkmann, B. G. et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595 (2008).
Franklin, R. J. & ffrench-Constant, C. Remyelination in the CNS: From biology to therapy. Nature Reviews Neuroscience 9, 839–855 (2008).
Nave, K. A. & Salzer, J. L. Axonal regulation of myelination by neuregulin 1. Current Opinion in Neurobiology 16, 492–500 (2006).
Poliak, S. & Peles, E. The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience 4, 968–980 (2003).
Sherman, D. L. & Brophy, P. J. Mechanisms of axon ensheathment and myelin growth. Nature Reviews Neuroscience 6, 683–690 (2005).
Siffrin, V. et al. Multiple sclerosis — candidate mechanisms underlying CNS atrophy. Trends in Neurosciences 33, 202–210 (2010).
Susuki, K. & Rasband, M. N. Molecular mechanisms of node of Ranvier formation. Current Opinion in Cell Biology 20, 616–623 (2008).
Waxman, S. G., Kocsis, J. D. & Stys, P. K., eds. The Axon: Structure, Function and Pathophysiology. New York: Oxford University Press, 1995.



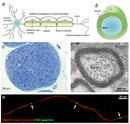
 Figure 1
Figure 1