« Prev Next »
From biology courses in school, many of us know that mitochondria are the organelles in charge of providing energy to the cells in our bodies. They provide energy through their ability to convert glucose into adenosine triphosphate, or ATP, which is the main energy source for cellular functions. Interestingly, scientists have learned that mitochondria evolved from bacteria a long, long time ago. Due to their similarities to bacteria, mitochondria can actually trigger severe illness in humans. How can organelles inside our bodies suddenly become a threat?
The Origin of Mitochondria as Cellular Organelles: Endosymbiotic Theory
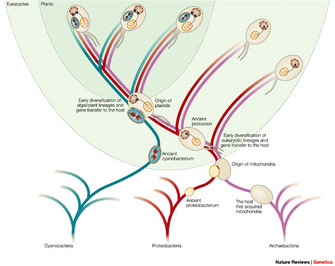
In 1967, Lynn Margulis (then Lynn Sagan) gathered diverse microbiological observations to support what is now known as the endosymbiotic theory. Her publication is now a landmark paper on the origin of eukaryotic cells (cells with a nucleus, like those of plants and animals). However, at the time, her article was rejected by fifteen scientific journals before it was accepted for publication in the Journal of Theoretical Biology. According to the endosymbiotic theory proposed by Margulis, mitochondria evolved from ancient symbiotic prokaryotes (organisms without nuclei, such as bacteria) that were absorbed into other free-living prokaryotes (Sagan 1967) (Figure 1). As you might suspect from the problems Margulis faced when trying to publish her article, this theory did not immediately receive wide acceptance. Indeed, it took decades for mainstream scientists to accept her hypothesis and begin calling it a theory.
The Evidence
What evidence did Margulis use to support her idea? Remember that at that time, scientists could examine cells under the microscope, lyse them, and study the chemistry of cellular components, but they could not examine DNA sequences. Margulis used a number of pieces of evidence to support her theory. She noted that mitochondria are self-replicating bodies. Mitochondria are surrounded by two or more membranes, and the innermost of these membranes is very similar in composition to bacteria. Both mitochondria and chloroplast have their own DNA, which by then was known to contain the hereditary material of organisms. Mitochondrial DNA has a simple circular structure, which is structurally similar to bacteria DNA and is more or less the same size. Mitochondrial ribosomes, enzymes, and transport systems are all similar to those of bacteria. Moreover, mitochondria are approximately the same size as bacteria.
With the advent of molecular biology methodologies, the amount of evidence supporting the endosymbiotic theory has grown. For example, by using genomic sequencing and phylogenetic analysis, scientists have shown that mitochondrial DNA share similar structural motifs with bacterial DNA and that mitochondrial genes originated within proteobacteria (a group of gram-negative bacteria that share common ribosomal RNA sequences) (Gray, Burger, & Lang 2001; Andersson et al. 2003). In an exciting recent development, the endosymbiotic theory crossed fields of knowledge to help physicians solve a mystery that they had been observing for years in the emergency room. What follows is the story of how mitochondria are related to immune inflammatory responses in critical care patients.
A Mystery in the Emergency Room: Mitochondria and the Inflammatory Response
For years, physicians working in critical care units have observed similarities between two different phenomena: systemic inflammatory response syndrome (SIRS) and sepsis. Patients who survive a severe trauma or physical injury may develop SIRS, a complication that can be life-threatening. SIRS is characterized by a fever, increased heart rate, and low blood pressure, resulting in generalized shock and compromised function of several organs. Sepsis, on the other hand, is a well-characterized phenomenon that occurs during the systemic inflammatory response to severe infection.
Physicians noted that SIRS and sepsis had many of the same symptoms, and both conditions showed clinical similarities. But what was the physiological basis for these similarities? At first glance, the two conditions seem to be very different. In SIRS, the inflammatory response — the body's defense to any type of lesion — is triggered by a physical injury or trauma. Meanwhile, the response in sepsis is caused by infections by diverse pathogens. Scientists proposed that the innate immune system recognizes certain molecules or "stimulators" through specialized receptors known as pattern-recognition receptors (PRRs), leading to molecular signaling pathways that result in an inflammatory response (Iwasaki & Medzhitov 2010). So, what are the "stimulators" in sepsis and in SIRS?
Scientists have now discovered that the innate immune system uses PRRs as "microbial sensors" to detect a set of evolutionarily conserved molecules found in a variety of pathogens. These molecules are collectively known as pathogen-associated molecular patterns (PAMPs), and they are expressed in a wide variety of microorganisms, including those that do not cause disease. In patients with severe infections such as sepsis, PAMPs are the major external "stimulators" of the inflammatory response
In 1994, Polly Matzinger proposed that the immune system does not only respond to pathogens, but it also responds to intracellular alarms that are activated when endogenous molecules are released in the body (Matzinger 1994). In the following years, scientists have experimentally shown that several endogenous molecules are released from damaged tissues, and these molecules were collectively named damage-associated molecular patterns (DAMPs). DAMPs are capable of initiating an inflammatory response similar to that produced by PAMPs (Lotze et al. 2007), even when there are no microbial infections present. So, why are the signaling pathways triggered by PAMPs in sepsis (external "stimulators") and DAMPs in SIRS (internal or endogenous "stimulators") so similar, and do these pathways overlap?
It turns out that our knowledge of mitochondria — that they were evolutionarily derived from bacteria and share conserved structural motifs with prokaryotes — can explain why the signaling pathways activated by external and internal triggers are so similar. With this idea in mind idea, Qin Zhang and his colleagues proposed a new and intriguing hypothesis: They suggested that mitochondrial DNA and proteins may act as DAMPs, triggering the same pathways activated by bacterial PAMPs. This hypothesis would explain the similarities observed between the immune response to infection and the immune response to trauma (Zhang et al. 2010).
Mitochondria and Bacteria Use the Same Mechanisms to Trigger Immune Responses
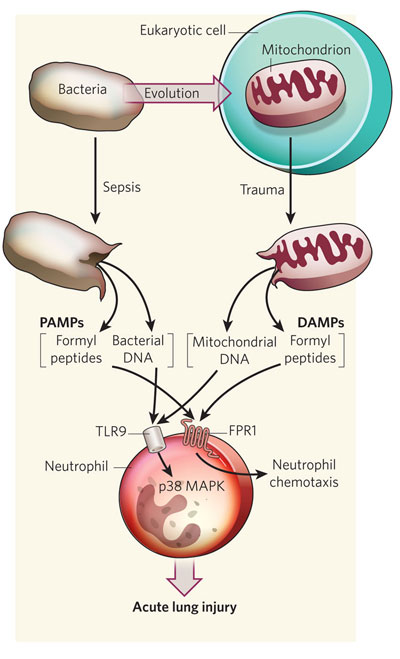
Where do these mitochondria come from? It is very likely that injuries cause cell lysis, degradation, and tissue breakdown (necrosis), which release the contents of cells, including broken mitochondria. Because each cell contains dozens of mitochondria and there are thousands of cells within our tissues, severe trauma can result in the release of large amounts of mitochondrial DAMPs into the blood.
In another experiment, Zhang's group analyzed how mitochondrial-derived DAMPs activate the immune response. Only bacteria and mitochondria have their proteins N-formylated; therefore, they are the only known sources of N-formyl peptides in nature. Formyl peptides can attract neutrophils, a type of white blood cell that is essential for the innate immune system. They can also activate neutrophils by specifically binding to the formyl peptide receptor-1 (FPR1), which is found on the surface of these cells. The activation of neutrophils promotes the inflammatory response by releasing chemical mediators and activating several enzymes known as MAP kinases. Using mitochondrial-derived DAMPs, the scientists were able to activate FPR1 and MAP kinases, which confirmed the presence of formyl peptides in mitochondrial DAMPs. Mitochondrial DNA can also bind to neutrophils through a specific receptor called the toll-like receptor 9 (TLR9) located on their surfaces. TLR9 is a member of the PRRs. By binding to TLR9, mitochondrial DNA can also activate the MAP kinases (). Zhang's group concluded that the immune response to injury "mimic sepsis" because mitochondrial DAMPs activate neutrophils through PRRs and FPR1, which normally would be activated by bacterial PAMPs.
Perhaps the most interesting question Zhang and his colleagues asked was if circulating mitochondrial DAMPs could cause neutrophil-mediated organ injury. To answer this question, they intravenously injected mitochondrial DAMPs into rats to see if they could produce organ injury in vivo. After exposure to the DAMPs, the animals were sacrificed, and samples of their organs were stained and observed under the microscope. They found that mitochondrial DAMPs produced systemic inflammation in several tissues, including a "marked-inflammatory lung injury," which is a major cause of respiratory failure in critically ill patients (such as those with severe trauma). In contrast, the control rats showed no evidence of inflammation.
In scientific research, a new discovery often opens the door to even more questions. Are there additional DAMPs associated with mitochondrial components that can trigger an immune response to trauma? Does the quantity of mitochondrial DAMPs released after trauma determine a patient's clinical outcome? In addition, can high amounts of circulating DAMPs be used as a marker to predict the severity of the inflammatory response and mortality?
Summary
Mitochondria are not only the "powerhouses" of our cells, they also play a significant role in triggering severe illnesses. Zhang and his colleagues provided evidence indicating that mitochondria are the missing link to explain the observed similarities between SIRS and sepsis. Severe trauma releases mitochondrial DAMPs into the blood where they are recognized by innate immunity through PRRs and FPR1, which also sense bacteria. Because mitochondrial DAMPs have evolutionarily conserved similarities to bacterial PAMPs, the release of mitochondrial DAMPs results in a "sepsis-like" state. This new model provides clues to better understand how the systemic inflammatory response develops. What scientists still do not know is if both SIRS and sepsis can be alleviated or prevented by inhibiting these pathways with pharmaceutical compounds. More research will be needed to answer these questions.
References and Recommended Reading
Andersson, S. G. et al. On the origin of mitochondria: A genomics perspective. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 358, 165–177 (2003).
Gray, M. W., Burger, G. & Lang, B. F. The origin and early evolution of mitochondria. Genome Biology, 2(6), reviews1018.1–1018.5 (2001)
Haslbrunner, E., Tuppy, H. & Schatz, G. Deoxyribonucleic acid associated with yeast mitochondria. Biochemical and Biophysical Research Communications 15, 127–132 (1964) .
Iwasaki, A. & Medzhitov, R. Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 (2010).
Lotze, M. T. et al. The grateful dead: Damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological Reviews 220, 60–81 (2007).
Matzinger, P. Tolerance, danger, and the extended family. Annual Review of Immunology 12, 991–1045 (1994).
Nass, M. M. & Nass, S. Intramitochondrial fibers with DNA characteristics. Journal of Cell Biology 19, 593–629 (1963)
Sagan, L. On the origin of mitosing cells. Journal of Theoretical Biology 14(3), 255–274 (1967).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–108 (2010)



 Figure 1: Endosymbiosis
Figure 1: Endosymbiosis


























