« Prev Next »
In recent years, however, researchers have increasingly turned to newer cytogenetic techniques. One such method is fluorescence in situ hybridization (FISH), a technique that uses fluorescently labeled probes to locate the positions of specific DNA sequences on chromosomes. Yet another popular technique is comparative genomic hybridization (CGH), which provides an alternative means of genome-wide screening for copy number variations. First developed to detect copy number changes in solid tumors, CGH uses two genomes, a test and a control, which are differentially labeled and competitively hybridized to metaphase chromosomes. The fluorescent signal intensity of the labeled test DNA relative to that of the reference DNA can then be linearly plotted across each chromosome, allowing the identification of copy number changes (Kallioniemi et al., 1992).
Unlike traditional techniques used to detect copy number gains and losses, which rely on the examination of a single target and prior knowledge of the region under investigation, CGH can be used to quickly scan an entire genome for imbalances. In addition, CGH does not require cells that are undergoing division (Speicher et al., 1993). However, as with earlier cytogenetic methods, the resolution of CGH has been limited to alterations of approximately 5-10 Mb for most clinical applications (Lichter et al., 2000; Kirchhoff et al., 1998).
Combining CGH with Microarrays: The Development of Array CGH
In an attempt to overcome some of the aforementioned limitations associated with traditional CGH, investigators have developed a newer method that combines the principles of CGH with the use of microarrays (Schena et al., 1995). Instead of using metaphase chromosomes, this method—which is known as array CGH, or simply aCGH—uses slides arrayed with small segments of DNA as the targets for analysis (Lucito et al., 2003). These microarrays are created by the deposit and immobilization of small amounts of DNA (known as probes) on a solid support, such as a glass slide, in an ordered fashion. Probes vary in size from oligonucleotides manufactured to represent areas of interest (25–85 base pairs) to genomic clones such as bacterial artificial chromosomes (80,000–200,000 base pairs). Because probes are several orders of magnitude smaller than metaphase chromosomes, the theoretical resolution of aCGH is proportionally higher than that of traditional CGH. The level of resolution is determined by considering both probe size and the genomic distance between DNA probes. For example, a microarray with probes selected from regions across the genome that are 1 Mb apart will be unable to detect copy number changes of the intervening sequence.
Regardless of the type of probe, the basic methodology for aCGH analysis is consistent (Figure 1). First, DNA is extracted from a test sample (e.g., blood, skin, fetal cells). The test DNA is then labeled with a fluorescent dye of a specific color, while DNA from a normal control (reference) sample is labeled with a dye of a different color. The two genomic DNAs, test and reference, are then mixed together and applied to a microarray. Because the DNAs have been denatured, they are single strands; thus, when applied to the slide, they attempt to hybridize with the arrayed single-strand probes. Next, digital imaging systems are used to capture and quantify the relative fluorescence intensities of the labeled DNA probes that have hybridized to each target. The fluorescence ratio of the test and reference hybridization signals is determined at different positions along the genome, and it provides information on the relative copy number of sequences in the test genome as compared to the normal genome. The recent sequencing of the human genome and development of high-throughput methods of robotically arraying genetic material on a solid surface have enabled the detection of submicroscopic chromosomal deletions and duplications at an unprecedented level (DeRisi et al., 1996; Schena et al., 1995; Shaffer et al., 2007).
Advantages of aCGH Technology
The primary advantage of aCGH is the ability to simultaneously detect aneuploidies, deletions, duplications, and/or amplifications of any locus represented on an array; in fact, one assay using this technique is equivalent to thousands of FISH experiments, with the attendant savings in labor and expense. In addition, aCGH has proven to be a powerful tool for the detection of submicroscopic chromosomal abnormalities in individuals with idiopathic mental retardation and various birth defects. Indeed, several large-scale studies demonstrate that aCGH has a 10%–20% detection rate of chromosomal abnormalities in children with mental retardation/developmental delay with or without congenital anomalies; only 3%–5% of these abnormalities would be detectable by other means. For example, in a study of 8,789 cases analyzed by aCGH, 1,049 (11.9%) had a clinically relevant chromosomal abnormality (Shaffer et al., 2007).
Studying Specific Chromosomal Regions with aCGH
Because aCGH facilitates simultaneous detection of multiple abnormalities and offers higher resolution than traditional cytogenetic methods, it has allowed investigators to focus on various types of rearrangements in particular regions of chromosomes. In recent years, aCGH has been particularly useful in the study of subtelomeric and pericentromeric rearrangements.
Subtelomeric Rearrangements
The largest study of subtelomeric abnormalities to date examined 11,688 cases with subtelomeric FISH and detected pathogenic abnormalities in 2.6% (Ravnan et al., 2006). Interestingly, recent large-scale prospective studies using aCGH on similar populations show that interstitial deletions (which are caused by two breaks in the chromosome arm, the loss of the intervening segment, and the rejoining of the chromosome segments) are two to three times more frequent than terminal imbalances in subtelomeric regions (Shaw-Smith et al., 2007).
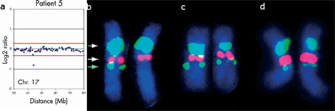
It is important to note that aCGH data can be verified using FISH analysis (Figure 2). For instance, Ballif and others (2007b) recently characterized 169 cases with subtelomeric abnormalities identified by aCGH. Although the coverage was sufficient to define the breakpoints in over half (56%) of the subtelomeric abnormalities, 44% of the abnormalities extended outside the coverage, suggesting that many such abnormalities are greater than 5 Mb in size. Of these 169 cases, 42 had interstitial deletions. These deletions would have been missed or incorrectly characterized by subtelomeric FISH panels that use a single clone to the most distal unique sequence for each region. In addition, six (3.5%) of the individuals had complex rearrangements that showed deletions along with duplications or additional deletions. The identification of these sorts of complex rearrangements suggests that chromosomal abnormalities are often more complex than previously thought.
Pericentromeric Rearrangements
Many such microdeletion syndromes are caused by nonallelic homologous recombination (NAHR) mediated by flanking segmental duplications (Shaffer et al., 2001). This mechanism predicts that reciprocal duplications of these deletions should occur with equal frequency (Lupski, 1998). However, duplications have been reported more rarely than expected. One explanation for this finding is that individuals with duplications usually have milder phenotypes than individuals with deletions, and these mild phenotypes may not lead to clinical investigation (Ensenauer et al., 2003; Yobb et al., 2005). Furthermore, duplications involving segments smaller than 1.5 Mb may be routinely missed even by FISH of interphase nuclei (Shaffer et al., 1997). However, recent large-population studies of individuals tested by aCGH have shown that the frequency of reciprocal duplications is higher than detected in previous studies that used other cytogenetic technologies (Shaffer et al., 2007; Lu et al., 2007). For example, duplications of the common Rett syndrome gene MECP2 have been identified in males with developmental delay (del Gaudio et al., 2006). In addition, the reciprocal duplications of microdeletion syndromes such as 3q29 microdeletion syndrome (Ballif et al., 2008a), Williams-Beuren syndrome (Kriek et al., 2006), and 22q11.21 microdeletion syndrome (Ensenauer et al., 2003) have also been identified by aCGH. The clinical significance of some of these reciprocal duplications is not yet known. For instance, only two individuals had de novo microduplications of 3q29, whereas the remaining cases were inherited from a carrier parent. Thus, the clinical significance of these duplications is unclear, and any phenotype may be modulated by an as-yet unidentified genetic modifier.
The Future of aCGH
Array CGH has propelled cytogenetics from the microscope to the computer, combining CGH with high-throughput microarrays to simultaneously analyze hundreds or thousands of discrete regions of the genome and identify unbalanced karyotypes. Array CGH combines the locus-specific nature of FISH with the global genome view of high-resolution chromosomes; thus, this method represents the integration of traditional and molecular cytogenetic techniques and will continue to enable the clinical diagnosis of chromosomal abnormalities at an unprecedented resolution in the years to come.
References and Recommended Reading
Ballif, B. C., et al. Monosomy 1p36 breakpoint junctions suggest pre-meiotic breakage-fusion-bridge cycles are involved in generating terminal deletions. Human Molecular Genetics 12, 2153–2165 (2003) doi:10.1093/hmg/ddg231 (link to article)
———. Translocation breakpoint mapping and sequence analysis in three monosomy 1p36 subjects with der(1)t(1;1)(p36;q44) suggest mechanisms for telomere capture in stabilizing de novo terminal rearrangements. Human Genetics 114, 198–206 (2004) doi:10.1007/s00439-003-1029-y
———. Discovery of a previously unrecognized microdeletion syndrome of 16p11.2-p12.2. Nature Genetics 39, 1071–1073 (2007a) (link to article)
———. The clinical utility of enhanced subtelomeric coverage in array CGH. American Journal of Medical Genetics 143A, 1850–1857 (2007b) doi:10.1002/ajmg.a.31842
———. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Molecular Cytogenetics 1, 1–7 (2008a) doi:10.1186/1755-8166-1-8
———. Identification of a previously unrecognized microdeletion syndrome of 16q11.2q12.2. Clinical Genetics (in press) (2008b) doi:10.1111/j.1399-0004.2008.01094.x
Barber, J. C. K., et al. 8p23.1 duplication syndrome: A novel genomic condition with unexpected complexity revealed by array CGH. European Journal of Human Genetics 16, 18–27 (2008) doi:10.1038/sj.ejhg.5201932 (link to article)
Berg, J. S., et al. Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genetics in Medicine 9(7), 427–441 (2007)
Biesecker, L. G. The end of the beginning of chromosome ends. American Journal of Medical Genetics 107, 263–266 (2002) doi:10.1002/ajmg.10160
Borozdin, W., et al. Detection of heterozygous SALL1 deletions by quantitative real time PCR proves the contribution of a SALL1 dosage effect in the pathogenesis of Townes-Brocks syndrome. Human Mutation 27, 211–212 (2006) doi:10.1002/humu.9396 (link to article)
Brewer, C., et al. A chromosomal deletion map of human malformations. American Journal of Human Genetics 63, 1153–1159 (1998)
del Gaudio, D., et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genetics in Medicine 8, 784–792 (2006)
DeRisi, J., et al. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nature Genetics 14, 457–460 (1996) doi:10.1038/ng1296-457 (link to article)
de Vries, B. B. A., et al. Diagnostic genome profiling in mental retardation. American Journal of Human Genetics 77, 606–616 (2005)
Ensenauer, R. E., et al. Microduplication 22q11.2, an emerging syndrome: Clinical, cytogenetic, and molecular analysis of thirteen patients. American Journal of Human Genetics 73, 1027–1040 (2003)
Flint, J., et al. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nature Genetics 9, 132–140 (1995) doi:10.1038/ng0295–132 (link to article)
Hassed, S. J., et al. A new genomic duplication syndrome complementary to the velocardiofacial (22q11 deletion) syndrome. Clinical Genetics 65, 400–404 (2004) doi:10.1111/j.0009-9163.2004.0212.x
Kallioniemi, A., et al. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. Science 258, 818–821 (1992) doi:10.1126/science.1359641
Kirchhoff, M., et al. Detection of chromosomal gains and losses in comparative genomic hybridization analysis based on standard reference intervals. Cytometry 31, 163–173 (1998)
———. High resolution comparative genomic hybridisation in clinical cytogenetics. Journal of Medical Genetics 38, 740–744 (2001) doi:10.1136/jmg.38.11.740
Knight, S. J., et al. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 354, 1676–1681 (1999)
Kriek, M., et al. Copy number variation in regions flanked (or unflanked) by duplicons among patients with developmental delay and/or congenital malformations; detection of reciprocal and partial Williams-Beuren duplications. European Journal of Human Genetics 14, 180–189 (2006) doi:10.1038/sj.ejhg.5201540 (link to article)
Lichter, P., et al. Comparative genomic hybridization: Uses and limitations. Seminars in Hematology 37, 348–357 (2000)
Lu, X., et al. Clinical implementation of chromosomal microarray analysis: Summary of 2513 postnatal cases. PLoS ONE 2, e327 (2007) doi:10.1371/journal.pone.0000327
Lucito, R., et al. Representational oligonucleotide microarray analysis: A high-resolution method to detect genome copy number variation. Genome Research 13, 2291–2305 (2003)
Lupski, J. R. Genomic disorders: Structural features of the genome can lead to DNA rearrangements and human disease traits. Trends in Genetics 14, 417–422 (1998) doi:10.1016/S0168-9525(98)01555-8
Pinkel, D., et al. High resolution analysis of DNA copy number variation using comparative genomic hybridization to microarrays. Nature Genetics 20, 207–211 (1998) doi:10.1038/2524 (link to article)
Potocki, L., et al. Molecular mechanism for duplication 17p11.2—The homologous recombination reciprocal of the Smith-Magenis microdeletion. Nature Genetics 24, 84–87 (2000) doi:10.1038/71743 (link to article)
———. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. American Journal of Human Genetics 80, 633–649 (2007)
Ravnan, J. B., et al. Subtelomere FISH analysis of 11,688 cases: An evaluation of the frequency and pattern of subtelomere rearrangements in individuals with developmental disabilities. Journal of Medical Genetics 43, 478–489 (2006) doi:10.1136/jmg.2005.036350
Saccone, S., et al. The highest gene concentrations in the human genome are in telomeric bands of metaphase chromosomes. Proceedings of the National Academy of Sciences 89, 4913–4917 (1992)
Schena, M., et al. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270, 467–470 (1995) doi:10.1126/science.270.5235.467
Shaffer, L. G., & Lupski, J. R. Molecular mechanisms for constitutional chromosomal rearrangements in humans. Annual Review of Genetics 34, 297–329 (2000)
Shaffer, L. G., et al. Diagnosis of CMT1A duplications and HNPP deletions by interphase FISH: Implications for testing in the cytogenetics laboratory. American Journal of Medical Genetics 69, 325–331 (1997)
———. Molecular cytogenetics of contiguous gene syndromes: Mechanisms and consequences of gene dosage imbalance. In Metabolic and Molecular Basis of Inherited Disease, 8th ed., ed. C. R. Scriver, et al. (New York, McGraw Hill, 2001), vol. 1, p. 1291.
———. The identification of microdeletion syndromes and other chromosome abnormalities: Cytogenetic methods of the past, new technologies for the future. American Journal of Medical Genetics 145C, 335–345 (2007) doi:10.1002/ajmg.c.30152
Shaw-Smith, C., et al. Microarray based comparative genomic hybridisation (array-CGH) detects submicroscopic chromosomal deletions and duplications in patients with learning disability/mental retardation and dysmorphic features. Journal of Medical Genetics 41, 241–248 (2004) doi:10.1136/jmg.2003.017731
She, X., et al. The structure and evolution of centromeric transition regions within the human genome. Nature 430, 857–864 (2004) doi:10.1038/nature02806 (link to article)
Solinas-Toldo, S., et al. Matrix-based comparative genomic hybridization: Biochips to screen for genomic imbalances. Genes, Chromosomes, and Cancer 20, 399–407 (1997)
Somerville, M. J., et al. Severe expressive-language delay related to duplication of the Williams-Beuren locus. New England Journal of Medicine 353, 1694–1701 (2005)
Speicher, M. R., et al. Molecular cytogenetic analysis of formalin-fixed, paraffin-embedded solid tumors by comparative genomic hybridization after universal DNA-amplification Human Molecular Genetics 2, 1907–1914 (1993)
Vissers, L. E., et al. Array-based comparative genomic hybridization for the genome-wide detection of submicroscopic chromosomal abnormalities. American Journal of Human Genetics 73, 1261–1270 (2003)
Yobb, T. M., et al. Microduplication and triplication of 22q11.2: A highly variable syndrome. American Journal of Human Genetics 76, 865–876 (2005)



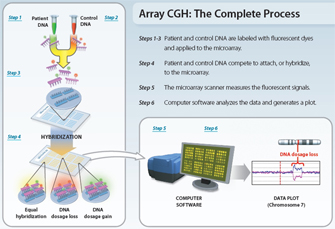
 Figure 1: Diagram of the microarray-based comparative genomic hybridization (aCGH) process.
Figure 1: Diagram of the microarray-based comparative genomic hybridization (aCGH) process.


























