« Prev Next »
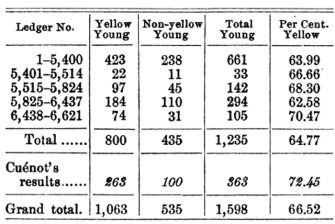
In 1905, Lucien Cuénot observed unusual patterns when studying inheritance of a coat color gene in mice. After mating two yellow mice, he observed that the offspring never showed a normal 3:1 phenotypic ratio. Instead, Cuénot always observed a 2:1 ratio, with two yellow mice for every one non-yellow mouse (Cuénot, 1905; Paigen, 2003). Cuénot thus determined that yellow coat color was the dominant phenotypic trait, and by using test crosses, he showed that all his yellow mice were heterozygotes. However, from his many crosses, Cuénot never produced a single homozygous yellow mouse. How could this be?
Shortly thereafter, in 1910, W. E. Castle and C. C. Little confirmed Cuénot's unusual segregation ratios (Figure 1). Moreover, they demonstrated that Cuénot's crosses resulted in what appeared to be non-Mendelian ratios because he had discovered a lethal gene. Castle and Little did this by showing that one-quarter of the offspring from crosses between heterozygotes died during embryonic development (Castle & Little, 1910; Paigen, 2003). This was why Cuénot never observed homozygous yellow mice! Thus, by considering embryonic lethality, or death, as a new phenotypic class, the classic 1:2:1 Mendelian ratio of genotypes could be reestablished (Figure 2).
As these examples illustrate, lethal genes cause the death of the organisms that carry them. Sometimes, death is not immediate; it may even take years, depending on the gene. In any case, if a mutation results in lethality, then this is indicative that the affected gene has a fundamental function in the growth, development, and survival of an organism.
Lethal genes can be recessive, as in the aforementioned mouse experiments. Lethal genes can also be dominant, conditional, semilethal, or synthetic, depending on the gene or genes involved. The following sections explore these variations in detail.
Recessive Lethal Genes
In 1907, Edwin Baur began his work with the snapdragon plant Antirrhinum and characterized the condition aurea, in which plants had golden instead of green leaves (Baur, 1907; Castle & Little, 1910). When two aurea snapdragon plants were crossed, Baur observed a 2:1 ratio of green seedlings to yellow seedlings. Homozygous aurea plants lacked normal chlorophyll development and died either during the embryonic stage or when the plant seedlings were two to three days old. In other words, like Cuénot's homozygous mice, the homozygous aurea plants could not fully develop, so an entire class of progeny died (Castle & Little, 1910).
Cuénot and Baur discovered these first recessive lethal genes because they altered Mendelian inheritance ratios. Recessive lethal genes can code for either dominant or recessive traits, but they do not actually cause death unless an organism carries two copies of the lethal allele. Examples of human diseases caused by recessive lethal alleles include cystic fibrosis, sickle-cell anemia, and achondroplasia. Achondroplasia is an autosomal dominant bone disorder that causes dwarfism. While the inheritance of one achondroplasia allele can cause the disease, the inheritance of two recessive lethal alleles is fatal.
Dominant Lethal Genes
Dominant lethal genes are expressed in both homozygotes and heterozygotes. But how can alleles like this be passed from one generation to the next if they cause death? Dominant lethal genes are rarely detected due to their rapid elimination from populations. One example of a disease caused by a dominant lethal allele is Huntington's disease, a neurological disorder in humans, which reduces life expectancy. Because the onset of Huntington's disease is slow, individuals carrying the allele can pass it on to their offspring. This allows the allele to be maintained in the population. Dominant traits can also be maintained in the population through recurrent mutations or if the penetrance of the gene is less than 100%.
Conditional Lethal Genes
Favism is a sex-linked, inherited condition that results from deficiency in an enzyme called glucose-6-phosphate dehydrogenase. It is most common among people of Mediterranean, African, Southeast Asian, and Sephardic Jewish descent (Allison, 1960). The disease was named because when affected individuals eat fava beans, they develop hemolytic anemia, a condition in which red blood cells break apart and block blood vessels. Blockage can cause kidney failure and result in death (Bowman & Walker, 1961). Affected individuals may also develop anemia when administered therapeutic doses of antimalarial medications and other drugs (Allison, 1960). Note, however, that the defective glucose-6-phosphate dehydrogenase allele only causes death under certain conditions, which makes it a conditional lethal gene. But why would this allele be so common? The interesting thing about individuals with the favism allele is that they are resistant to malaria, because it is more difficult for malaria parasites to multiply in cells with deficient amounts of glucose-6-phosphate dehydrogenase. Therefore, carrying the allele for favism confers an intrinsic genetic or adaptive advantage by protecting individuals from contracting malaria.
Conditional lethal genes can also be expressed due to specific circumstances, such as temperature. For example, a mutant protein may be genetically engineered to be fully functional at 30°C and completely inactive at 37°C. Meanwhile, the wild-type protein is fully functional at both temperatures. The condition in which the mutant phenotype is expressed is termed nonpermissive. Meanwhile, the condition in which the wild-type phenotype is expressed is called permissive. In order to study a conditional lethal mutant, the organism must be maintained under permissive conditions and then switched to the nonpermissive condition during the course of a specific experiment. By developing a conditional lethal version of a dominant lethal gene, scientists can study and maintain organisms carrying dominant lethal alleles.
Semilethal or Sublethal Genes
Hemophilia is a hereditary disease caused by deficiencies in clotting factors, which results in impaired blood clotting and coagulation. Because the allele responsible for hemophilia is carried on the X chromosome, affected individuals are predominantly males, and they inherit the allele from their mothers. Normally, clotting factors help form a temporary scab after a blood vessel is injured to prevent bleeding, but hemophiliacs cannot heal properly after injuries because of their low levels of blood clotting factors. Therefore, affected individuals bleed for a longer period of time until clotting occurs. This means that normally minor wounds can be fatal in a person with hemophilia. The alleles responsible for hemophilia are thus called semilethal or sublethal genes, because they cause the death of only some of the individuals or organisms with the affected genotype.
Synthetic Lethal Genes
Scientists studying the fruit fly observed that pairwise combinations of some mutant alleles were not viable, whereas singly, the same mutant alleles did not cause death (Boone et al., 2007). In other words, some mutations are only lethal when paired with a second mutation. These genes are called synthetic lethal genes. When the functions of the two affected genes are not fully understood, scientists can create and study synthetic lethal mutants and their phenotypes to identify a gene's function. Mechanisms can also be hypothesized from the known functions of pairs of mutated alleles.
For instance, if both mutations occur in nonessential genes, a scientist could hypothesize that the two genes function in parallel pathways that share information with one another. Each of the two pathways could compensate for a defect in the other, but when both pathways have a mutation, the combination results in synthetic lethality. Synthetic lethality can also indicate that two affected genes have the same role, and therefore, lethality only results when both copies are nonfunctional and one gene cannot substitute for the other. Additionally, both genes may function in the same essential pathway, and the pathway's function may be diminished by each mutation.
When an allele causes lethality, this is evidence that the gene must have a critical function in an organism. The discoveries of many lethal alleles have provided information on the functions of genes during development. Additionally, scientists can use conditional and synthetic lethal alleles to study the physiological functions and relationships of genes under specific conditions.
Exploring Genetic Interactions and Networks with Yeast
References and Recommended Reading
Allison, A. C. Glucose-6-phosphate dehydrogenase deficiency in red blood cells of East Africans. Nature 186, 531–532 (1960) doi:10.1038/186531a0 (link to article)
Baur, E. Untersuchungen über die Erblichkeitsverhältnisse einer nur in Bastardform lebensfähigen Sippe von Antirrhinum majus. Berichte der Deutschen Botanischen Gesellschaft 25, 442–454 (1907)
Boone, C., et al. Exploring genetic interactions and networks with yeast. Nature Reviews Genetics 8, 437–449 (2007) doi:10.1038/nrg2085 (link to article)
Bowman, J. E., & Walker, D. G. Action of Vicia faba on erythrocytes: Possible relationship to favism. Nature 189, 555–556 (1961) doi:10.1038/189555a0 (link to article)
Castle, W. E., & Little, C. C. On a modified Mendelian ratio among yellow mice. Science 32, 868–870 (1910)
Cuénot, L. Les races pures et leurs combinaisons chez les souris. Archives de Zoologie Experimentale et Generale 4, 123–132 (1905)
Link, G. K. K. The role of genetics in etiological pathology. Quarterly Review of Biology 7, 127–171 (1932)
Paigen, K. One hundred years of mouse genetics: An intellectual history. I. The classical period (1902-1980). Genetics 163, 1–7 (2003)



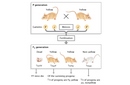
 Figure 2
Figure 2


























