« Prev Next »
If your parents, grandparents, or other relatives have had cancer, you have probably wondered at some point about your own risk of developing the same type of cancer(s). In fact, fewer than 5 percent of cancers are inherited, or "familial." Most cancers result from genetic mutations that occur during a person's own lifetime. Thus, when scientists say, "Cancer is a genetic disease," they usually are referring not to the mutations you inherit, but rather to the mutations that originate and accumulate in your cells over the course of your lifetime.
Another way of saying that cancer is genetic is saying that it is a disease caused by somatic mutations, or DNA mutations that have not been inherited and occur at some point after conception in somatic (body) cells. Even though this so-called somatic mutation theory of cancer was introduced in the 1920s, it wasn't until the 1960s that the concept started to dominate cancer research. In recent years, new evidence in the field of epigenetics has provided additional insight into cancer research, leading many scientists to argue that there is more to cancer than mutations alone; nonetheless, the somatic mutation theory remains the prevailing paradigm in cancer research. According to this theory, cancer is a genetic disease because most cancer cells do what they do — that is, proliferate at a rapid pace — as a result of mutations in their genetic sequence. Of course, not all mutations cause cancer. Mutations frequently occur as a normal part of cell life, and most have a negligible effect on phenotype. Scientists therefore suspect that most malignant transformations (the process that precancerous cells go through to become actual tumor cells) result from the accumulation of multiple mutations. But what might these exact mutations be?
Over the past forty years or so, scientists have sought to answer that very question, and they have identified dozens of different cancer-causing mutations. Most of these mutations occur in genes that play some kind of role in regulating the cell cycle, or the series of events that a cell goes through as it replicates its DNA and divides. Furthermore, the majority of these mutations were discovered in other species, like yeast, before their role in human cancer was realized.
Lessons from Baker’s Yeast
One of the first scientists to discover some of these cancer-causing mutations was a biologist named Leland H. Hartwell. Hartwell would eventually go on to receive a Nobel Prize in 2001, and he is still a leader in the field of cancer genetic research. The first in his family to attend college and driven, in part, by a desire to understand things, Hartwell enrolled as a physics major, but he eventually found his calling in the biology lab. In particular, he was interested in "the cancer problem": Why are cancer cells unable to control themselves? What goes wrong during their life cycle?
When it came time to start his own research program after receiving his doctorate, Hartwell decided to use an easy-to-manipulate, single-celled eukaryote as a model system for studying cancer and the cell cycle. This organism was Saccharomyces cerevisiae, or baker's yeast—the very same yeast people have been using for thousands of years to brew beer, bake bread, and make all sorts of other fermented foods and beverages. As it turns out (and as Hartwell discovered), the same genes that control the cell cycle in baker's yeast (and that malfunction in tumor cells) exist in more or less the same capacity in human cells.
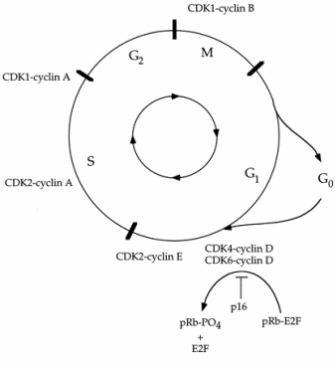
In an elegant series of experiments early in his career, Hartwell identified more than 100 genes involved in cell cycle control. In yeast, these genes are known as the CDC (cell division cycle) genes. Hartwell also determined the pathway of cell cycle regulation events; that is, he discovered which genes regulate which parts of the cell cycle. Many of the same genes that work to regulate cell division in yeast were also shown to work similarly in humans. The CDC genes that Hartwell found were shown to regulate the cell cycle by either stimulating or inhibiting cell division in response to the barrage of signals these cells constantly receive from their environment. However, in cancer cells, mutated genes that normally stimulate cell division only at the right time and place (so-called proto-oncogenes) start operating like stuck accelerators (turning into oncogenes). Meanwhile, other mutated genes that normally inhibit excessive cell division (so-called tumor suppressor genes) simply stop working, similar to broken brakes. Because of their role in regulating cell division so precisely, and because they are often found to be mutated in tumor cells, these genes are frequently often referred to as "cancer genes." Most oncogenes function as dominant mutations (i.e., only one mutated copy of the gene is needed for the gene to start operating like a stuck accelerator), whereas most tumor suppressor genes function as recessive mutations (i.e., two mutated copies of the gene are needed for the gene to lose its ability to apply the brakes). Despite this difference, both types of mutations—stuck accelerators and broken brakes—ultimately lead to out-of-control cell growth and reproduction. Thus, what starts as a single-cell malignancy eventually develops into a tumorous mass of cancerous cells.
In addition to determining the role of oncogenes and tumor suppressor genes, Hartwell also introduced the concept of cell cycle "checkpoints" and their related genes. Checkpoints are specific points during the cell cycle at which a cell "evaluates" its cell cycle progress. If the checkpoint molecular machinery detects any damage in the cell's DNA, cell cycling stops until either the damaged DNA is repaired by a so-called DNA repair gene (which is yet a third type of cancer gene) or the damaged cell self-destructs through a process known as apoptosis. Therefore, when a cell's checkpoint genes become mutated, which is often the case in cancer cells, the cells lose their capacity to stop dividing if their DNA is damaged. In fact, one of the most common cancer genes—p53—is a mutated checkpoint gene. Mutated DNA repair genes and checkpoint genes are like bad mechanics: The repair genes can't fix broken brakes or stuck accelerators, and checkpoint genes don't even notice that anything's wrong.
From Yeast to Humans
The universality of cell cycle controls in eukaryotes should have been anticipated given the high conservation already noted for biochemical pathways and for many processes of molecular biology. Possibly the rather different appearance of cells and cell division in microbial eukaryotes, plants, and Metazoa made universalities seem less likely than these other processes. However, Schwann, one of the early proponents of the cell theory, had already recognized this possibility in 1839 when he stated, ‘We have seen that . . . cells are formed and grow in accordance with essentially the same laws; hence, that these processes must everywhere result from the operation of the same forces.' (Wilson 1925)
Given the knowledge that Hartwell, Nurse, and countless other scientists have generated about the three types of genes that regulate the cell cycle — CDC/CDK genes, checkpoint genes, and DNA repair genes — the obvious question is, what comes next? The hope is that this knowledge will help scientists not only better understand cancer in humans (i.e., how mutations in cell cycle regulation lead to tumor formation), but also better prevent and treat cancer. In particular, as Hartwell currently advocates, this information could be used to develop more effective chemotherapy drugs—therapies that intervene with the cell cycle in humans in more precise ways and that can be tailored to particular types of cell cycle mutations. As Hartwell (who is still working with yeast) and his colleagues from the Fred Hutchinson Cancer Research Center in Seattle, Washington, have shown, the nature of the cell cycle checkpoint mutations that are present in a tumor in all likelihood affects the outcome of a cancer patient's therapy. In other words, all tumors have mutations, but some have different mutations than others; moreover, different cancer drugs work better on different tumors depending on the nature of the mutations in the tumor cells. So, the hope is that instead of administering "drug X" to everyone who has a particular type of tumor, doctors might someday be able to prescribe drug X only to those people whose tumor cells contain the specific cell cycle mutations that drug X is particularly effective at targeting. This approach, called pharmacogeneomics, will likely improve the overall outcomes of cancer therapy.
References and Recommended Reading
Hartwell, L. H. "Yeast and Cancer," in : The Nobel Prizes 2001, ed. Tore Frängsmyr (Stockholm, Nobel Foundation, 2002)
Nurse, P. M. "Cyclin-Dependent Kinases and Cell Cycle Control," in : The Nobel Prizes 2001, ed. Tore Frängsmyr (Stockholm, Nobel Foundation, 2002)
Simon, J., et al. Differential toxicities of anticancer agents among DNA repair and checkpoint mutations of Saccharomyces cerevisiae. Cancer Research 60, 328–333 (2000)
Wilson, E. The Cell in Development and Heredity (New York, Macmillan, 1925)



 Figure 1: Many of the mammalian cell cycle genes were first identified and studied in yeast.
Figure 1: Many of the mammalian cell cycle genes were first identified and studied in yeast.